Home /
Expert Answers /
Chemistry /
6-1-to-answer-the-questions-interpret-the-following-lewis-diagram-for-nhf2-1-for-the-central-n-pa554
(Solved): 6.1 To answer the questions, interpret the following Lewis diagram for NHF2. 1. For the central n ...
6.1
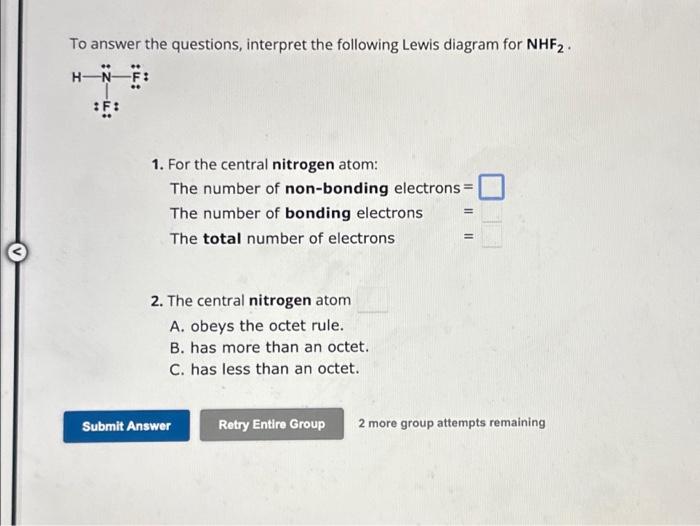

To answer the questions, interpret the following Lewis diagram for . 1. For the central nitrogen atom: The number of non-bonding electrons= The number of bonding electrons = The total number of electrons = 2. The central nitrogen atom A. obeys the octet rule. B. has more than an octet. C. has less than an octet. 2 more group attempts remaining
Expert Answer
A single line represents a single bond,which represents 2 electrons involved in the bond. Means each line around an atom represents 2 bonding electrons. Each dot on the atom represents a non bonding electronThere are 3 bonds and two dots around Nitrogen(N)Means For the central nitrogen atom: The number of non-bonding electrons=2The number of bonding electrons=