Home /
Expert Answers /
Chemistry /
52-use-vsepr-theory-to-predict-the-shape-of-these-molecules-a-mathrm-sif-4-tetrahedra-pa334
(Solved): 52. Use VSEPR theory to predict the shape of these molecules: (a) \( \mathrm{SiF}_{4} \) tetrahedra ...
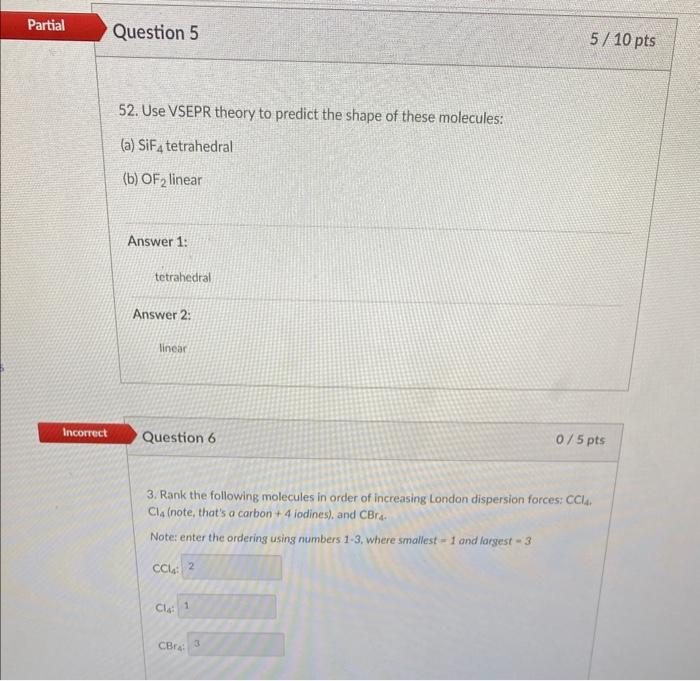
52. Use VSEPR theory to predict the shape of these molecules: (a) \( \mathrm{SiF}_{4} \) tetrahedral (b) \( \mathrm{OF}_{2} \) linear Answer 1: tetrahedral Answer 2: linear Question 6 \( 0 / 5 \mathrm{pts} \) 3. Rank the following molecules in order of increasing London dispersion forces: \( \mathrm{CCl}_{4 \text {. }}^{\text {. }} \). \( \mathrm{Cl}_{4} \) (note, that's a carbon \( +4 \) iodines), and \( \mathrm{CBr}_{4} \). Note: enter the ordering using numbers \( 1-3 \), where smallest \( =1 \) and largest \( -3 \) \[ \mathrm{CCl}_{4} \text { : } \] \( \mathrm{Cl}_{4} \) CBrat
Expert Answer
5) Shape of the molecules: i) SiF4 : It is tetrahedral as per VSEPR ii) OF2: As per VSEPR OF2 is linear