Home /
Expert Answers /
Biology /
5-the-structure-of-a-disaccharide-is-shown-below-which-statement-applies-a-both-rings-a-and-b-a-pa265
(Solved): 5. The structure of a disaccharide is shown below. Which statement applies? A. Both rings A and B a ...
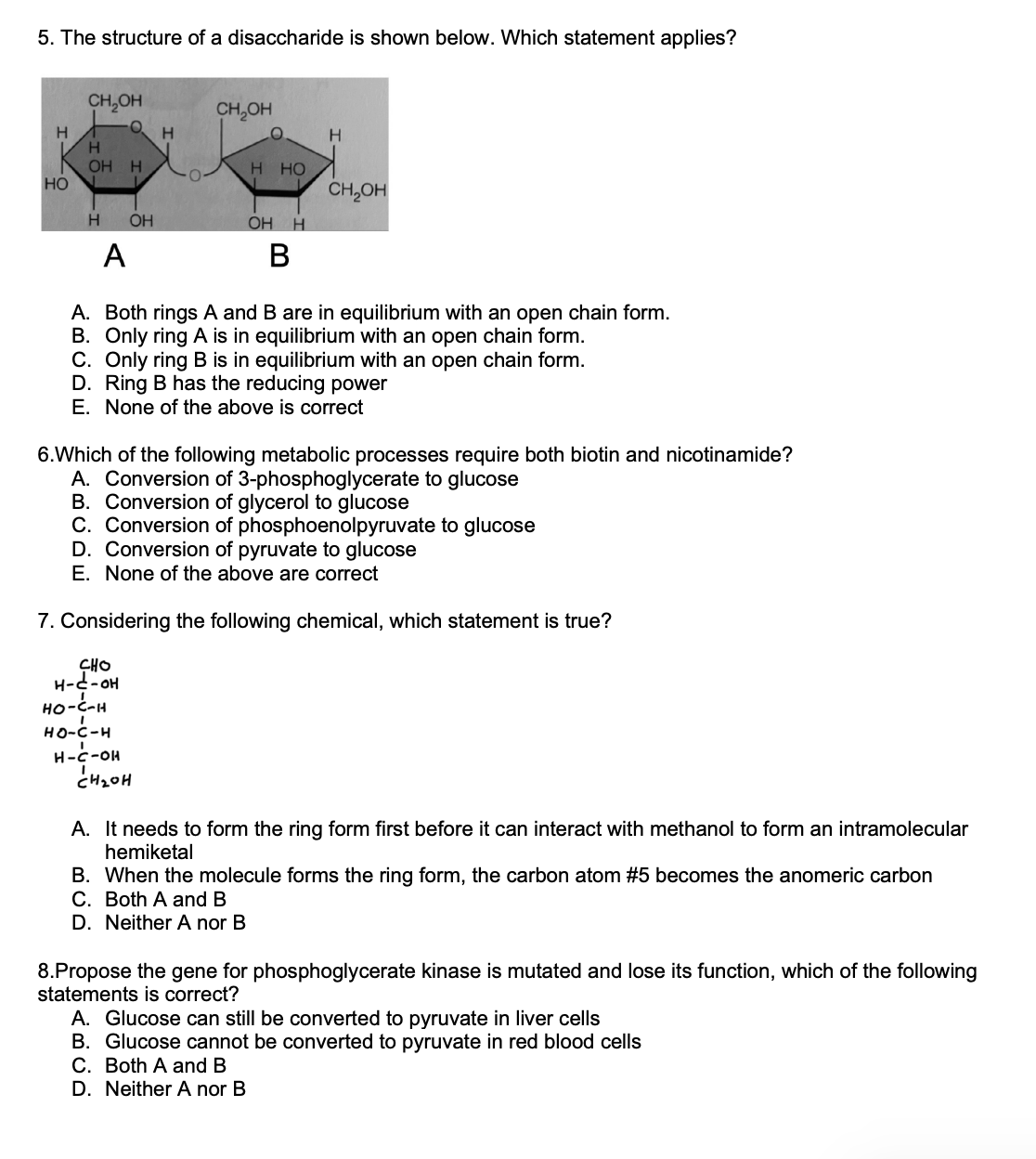
5. The structure of a disaccharide is shown below. Which statement applies? A. Both rings and are in equilibrium with an open chain form. B. Only ring is in equilibrium with an open chain form. C. Only ring is in equilibrium with an open chain form. D. Ring has the reducing power E. None of the above is correct 6.Which of the following metabolic processes require both biotin and nicotinamide? A. Conversion of 3-phosphoglycerate to glucose B. Conversion of glycerol to glucose C. Conversion of phosphoenolpyruvate to glucose D. Conversion of pyruvate to glucose E. None of the above are correct 7. Considering the following chemical, which statement is true? A. It needs to form the ring form first before it can interact with methanol to form an intramolecular hemiketal B. When the molecule forms the ring form, the carbon atom becomes the anomeric carbon C. Both and D. Neither A nor B 8.Propose the gene for phosphoglycerate kinase is mutated and lose its function, which of the following statements is correct? A. Glucose can still be converted to pyruvate in liver cells B. Glucose cannot be converted to pyruvate in red blood cells C. Both and D. Neither A nor B
Expert Answer
5. The correct answer is B. Only ring A is in equilibrium with an open chain form. In the given structure of the disaccharide, only ring A (the leftmost ring) is in equilibrium with an open chain form. This is indicated by the presence of an aldehyde functional group (shown as -CHO) in ring A. The open chain form of a sugar molecule typically exists in equilibrium with its cyclic form, where the aldehyde or ketone group reacts with a hydroxyl group within the same molecule, forming a cyclic structure. In this case, ring A can exist in both an open chain form and a cyclic form, while ring B (the rightmost ring) lacks the necessary functional groups to undergo such equilibration. Option A is incorrect because it suggests that both rings A and B are in equilibrium with an open chain form, which is not supported by the given structure. Option C is incorrect because it suggests that only ring B is in equilibrium with an open chain form, which is not supported by the given structure. Option D is incorrect because it states that ring B has reducing power. However, reducing power is associated with the presence of a free aldehyde or ketone group, which is found in ring A, not ring B.