Home /
Expert Answers /
Chemistry /
42-from-the-one-component-phase-diagram-shown-below-one-can-conclude-that-a-no-solid-exists-abo-pa275
(Solved): 42. From the one-component phase diagram shown below, one can conclude that (A) no solid exists abo ...
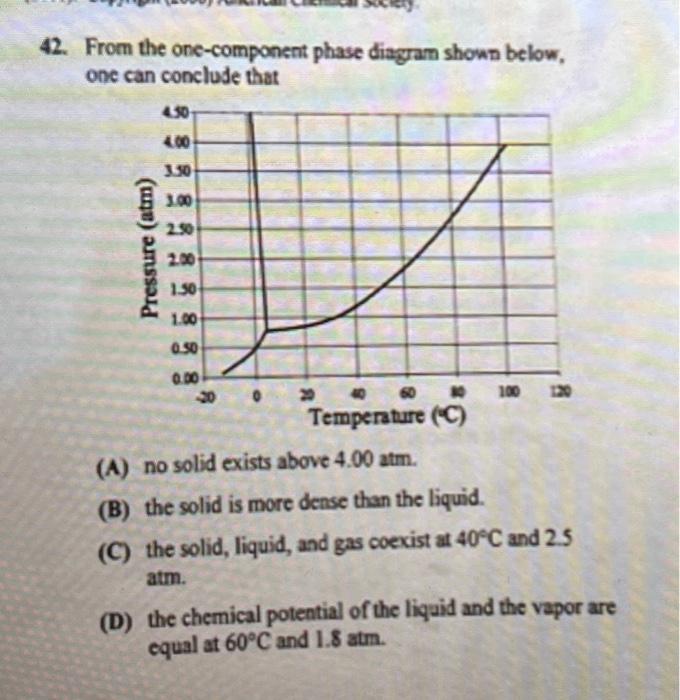
42. From the one-component phase diagram shown below, one can conclude that (A) no solid exists above . (B) the solid is more dense than the liquid. (C) the solid, liquid, and gas coexist at and 2.5 atm. (D) the chemical potential of the liquid and the vapor are equal at and .
Expert Answer
Answer A) - At pressures above 4.00 atom the substance is expected to exist as either a liquid or gas phase. The absence of a solid phase suggests that the substance remains in a liquid or gaseous state under these conditions.