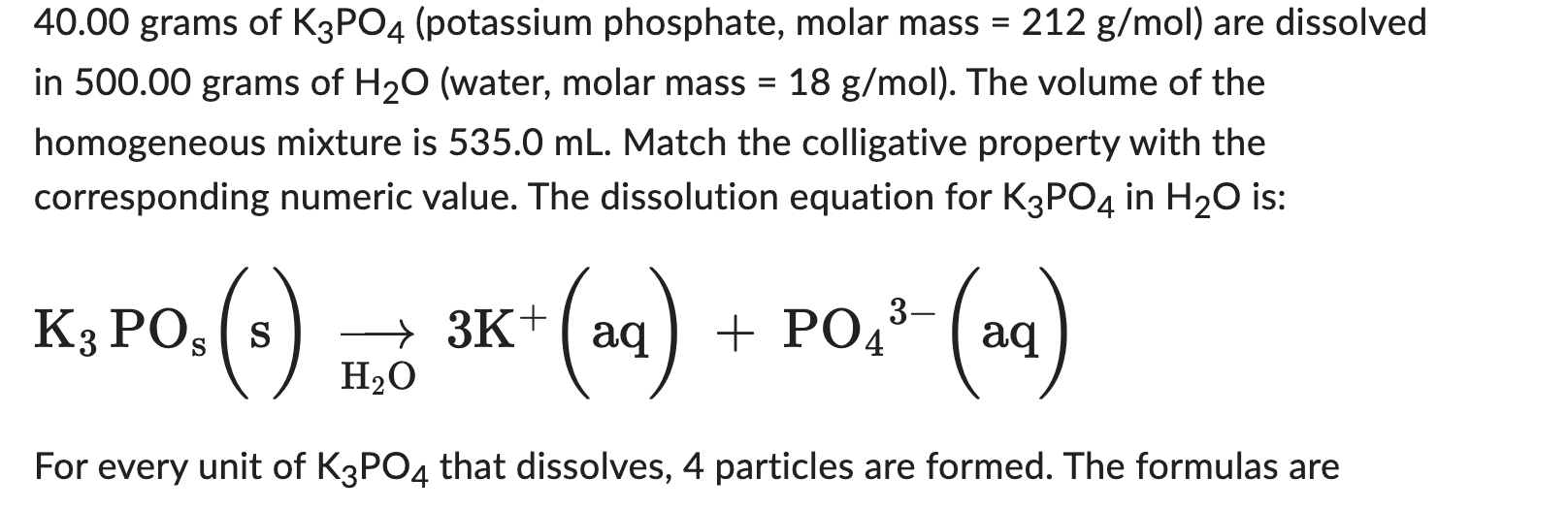Home /
Expert Answers /
Chemistry /
40-00-grams-of-k3po4-potassium-phosphate-molar-mass-212g-mol-are-dissolved-in-500-00-pa274
(Solved): 40.00 grams of K3PO4 (potassium phosphate, molar mass =212g/mol ) are dissolved in 500.00 ...
grams of (potassium phosphate, molar mass ) are dissolved in grams of (water, molar mass ). The volume of the homogeneous mixture is . Match the colligative property with the corresponding numeric value. The dissolution equation for in is: For every unit of that dissolves, 4 particles are formed. The formulas are
moles moles of solution
1. boiling point of solution, in 2. freezing point of solution, in 3. vapor pressure of solution at , in vapor pressure of pure 4. osmotic pressure of solution at , in atm
Expert Answer
Answer . Here given for K3PO4 in water solution