Home /
Expert Answers /
Chemistry /
4-parts-calculate-the-ph-and-poh-of-the-solutions-with-the-following-hydrogen-ion-or-hydroxide-ion-c-pa242
(Solved): 4 parts Calculate the pH and pOH of the solutions with the following hydrogen ion or hydroxide ion c ...
4 parts
![\[
\begin{array}{r}
{\left[\mathrm{OH}^{-}\right]=1.59 \times 10^{-9} M} \\
\mathrm{pH} \\
\mathrm{pOH}
\end{array}
\]
Choose](https://media.cheggcdn.com/study/159/15985553-b7ad-4292-a9ed-e7e1478bfd23/image)
![Part 4 (3 pts)
\[
\left[\mathrm{H}^{+}\right]=1.85 \times 10^{-4} \mathrm{M}
\]
\( \mathrm{pH} \)
\( \mathrm{pOH} \)
Choose o](https://media.cheggcdn.com/study/47b/47b0c44f-9ed5-473b-820d-f247d8859b67/image)

![\[
\begin{array}{r}
{\left[\mathrm{OH}^{-}\right]=1.59 \times 10^{-9} M} \\
\mathrm{pH} \\
\mathrm{pOH}
\end{array}
\]
Choose](https://media.cheggcdn.com/study/159/15985553-b7ad-4292-a9ed-e7e1478bfd23/image)
![Part 4 (3 pts)
\[
\left[\mathrm{H}^{+}\right]=1.85 \times 10^{-4} \mathrm{M}
\]
\( \mathrm{pH} \)
\( \mathrm{pOH} \)
Choose o](https://media.cheggcdn.com/study/47b/47b0c44f-9ed5-473b-820d-f247d8859b67/image)
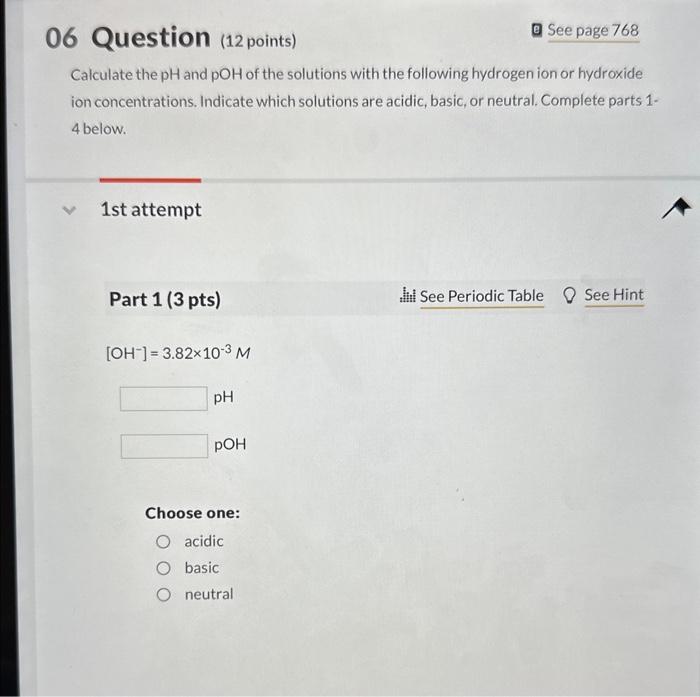
Calculate the and of the solutions with the following hydrogen ion or hydroxide ion concentrations. Indicate which solutions are acidic, basic, or neutral. Complete parts 1 4 below. 1 st attempt Part 1 (3 pts) Choose one: acidic
Choose one: acidic basic neutral Part 3 (3 pts) Choose one: acidic
Part 4 (3 pts) Choose one: acidic basic neutral
Expert Answer
Hydrogen ions (H+) and hydroxyl ions are produced when water (H2O) se