Home /
Expert Answers /
Chemistry /
4-for-the-equilibrium-br2-g-cl2-g-2brcl-g-the-equilibrium-constant-kp-is-7-0-at-pa917
(Solved): 4. For the equilibrium Br2(g)+Cl2(g)2BrCl(g), the equilibrium constant Kp is 7.0 at ...
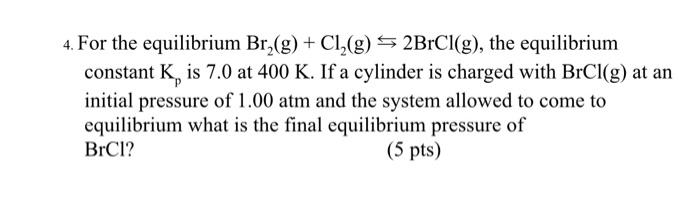
4. For the equilibrium , the equilibrium constant is 7.0 at . If a cylinder is charged with at an initial pressure of and the system allowed to come to equilibrium what is the final equilibrium pressure of
Expert Answer
given reaction Br 2( g)+Cl 2( g )? 2BrCl(g)initial 0 0 1.00change