Home /
Expert Answers /
Chemistry /
4-a-what-is-the-molality-of-a-solution-that-contains-13-4-grams-of-calcium-chloride-dissolved-in-6-pa717
(Solved): 4. a. What is the molality of a solution that contains 13.4 grams of calcium chloride dissolved in 6 ...
4. a. What is the molality of a solution that contains 13.4 grams of calcium chloride dissolved in 655 mL of water? ??=40.08 CIX2= 70.9 ?? 313.4 547 111.7 Answer: 0.184 m CaCl?2 C655ml 0.655 kg 3595. 107 b. What is the molality of the ions in the solution? Answer: 0.553 m CaCl? ions 110.98 110.98 1 0.655 ·x- €0.184 m. CaCl 2
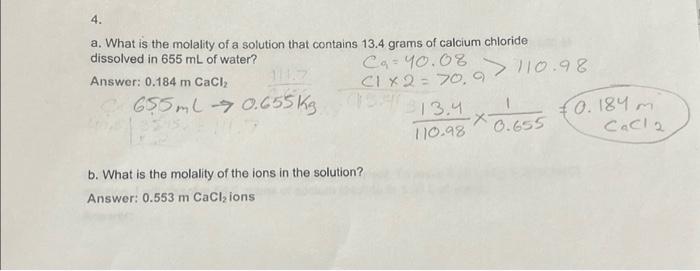
a. What is the molality of a solution that contains 13.4 grams of calcium chloride dissolved in of water? Answer: b. What is the molality of the ions in the solution? Answer: ions
Expert Answer
a. First, let's calculate the amount of calcium chloride (CaCl2) in moles:Mass of calcium chloride = 13.4 grams Molar mass of calcium chloride (CaCl2) = 110.98 g/molNumber of moles of calcium chloride = Mass / Molar mass = 13.4 g / 110.98 g/mol = 0.1207 mol