Home /
Expert Answers /
Chemistry /
3cm-3cm-4-cm-determine-the-number-of-significant-figures-certain-digits-and-uncertain-digits-pa699
(Solved): 3cm ||| 3cm 4 cm Determine the number of significant figures, certain digits, and uncertain digits ...
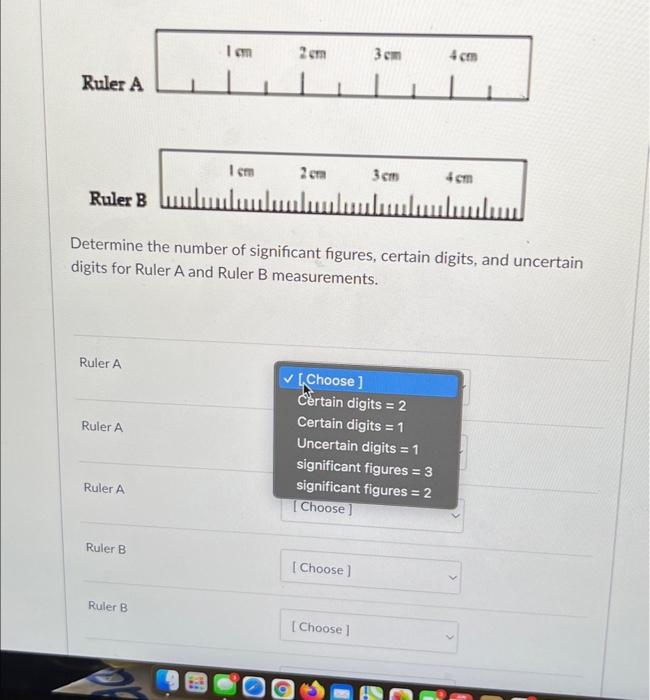

3cm ||| 3cm 4 cm Determine the number of significant figures, certain digits, and uncertain digits for Ruler A and Ruler B measurements. Ruler A ? [Choose ] Certain digits = 2 Certain digits = 1 Ruler A Uncertain digits = 1 significant figures = 3 significant figures = 2 [Choose] [Choose ] [Choose ] Ruler A Ruler A Ruler B 1 cm ???????????????? Ruler B Ruler B 2cm 2cm 4 cm
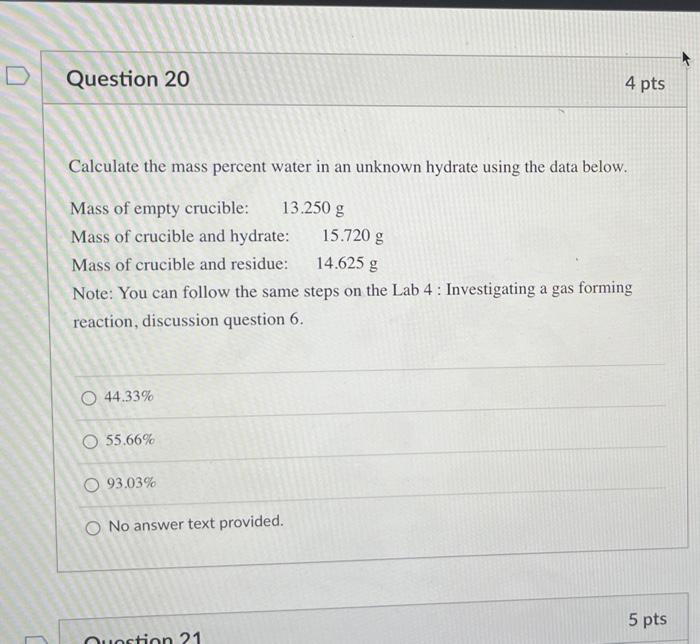
Question 20 4 pts Calculate the mass percent water in an unknown hydrate using the data below. Mass of empty crucible: 13.250 g Mass of crucible and hydrate: 15.720 g Mass of crucible and residue: 14.625 g Note: You can follow the same steps on the Lab 4: Investigating a gas forming reaction, discussion question 6. O 44.33% O 55.66% O93.03% O No answer text provided. 5 pts Jestion 21
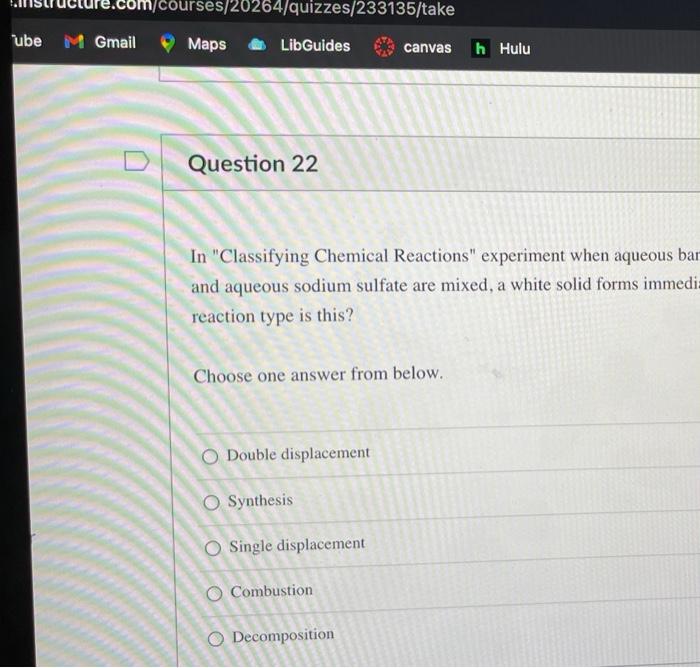
m/courses/20264/quizzes/233135/take Maps LibGuides canvas h Hulu Question 22 In "Classifying Chemical Reactions" experiment when aqueous bar and aqueous sodium sulfate are mixed, a white solid forms immedi- reaction type is this? Choose one answer from below. O Double displacement O Synthesis O Single displacement O Combustion O Decomposition ube M Gmail
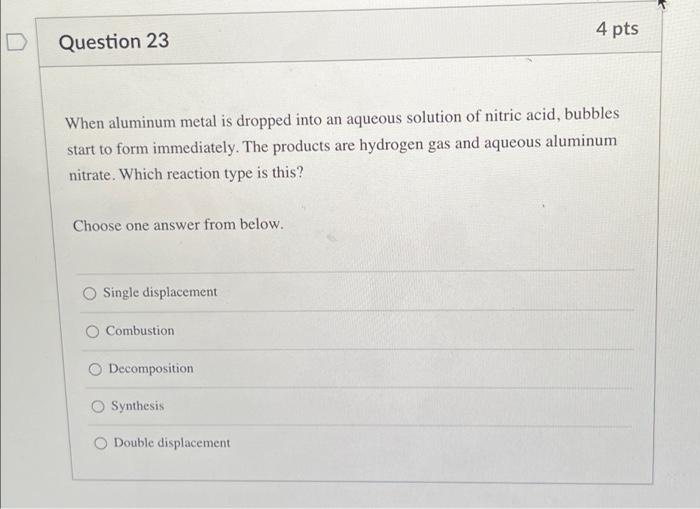
4 pts Question 23 When aluminum metal is dropped into an aqueous solution of nitric acid, bubbles start to form immediately. The products are hydrogen gas and aqueous aluminum nitrate. Which reaction type is this? Choose one answer from below. Single displacement O Combustion O Decomposition O Synthesis Double displacement
Question 28 5 pts Jacques Charles used this reaction to prepare hydrogen gas for his historic balloon flights: Fe(s) + H?SO4 (aq) ?? FeSO4 (aq) + H?(g) He wants to prepare a small test balloon to check flight conditions before he lifts off in his giant balloon. He has 119 g of iron and 258 ml of 12 M (mol/L.) sulfuric acid available. What is the maximum size his test balloon can be in L? The temperature in Paris is a chilly 18 °C and the atmospheric pressure is 770 mm Hg. (R=0.0821 L atm/mol K) Hint: this is a limiting reactant type problem. Note: You can follow the steps on the Lab 8: Investigating a gas forming reaction, discussion questions, question 5. O 50.2 L 02131 3.096 L 73.0L 0258 L DQuestion 29 4 pts