Home /
Expert Answers /
Chemistry /
3-the-decomposition-of-urea-nh2-2co-in-0-10mhcl-follows-the-equation-nh2-2co-aq-2-pa302
Expert Answer
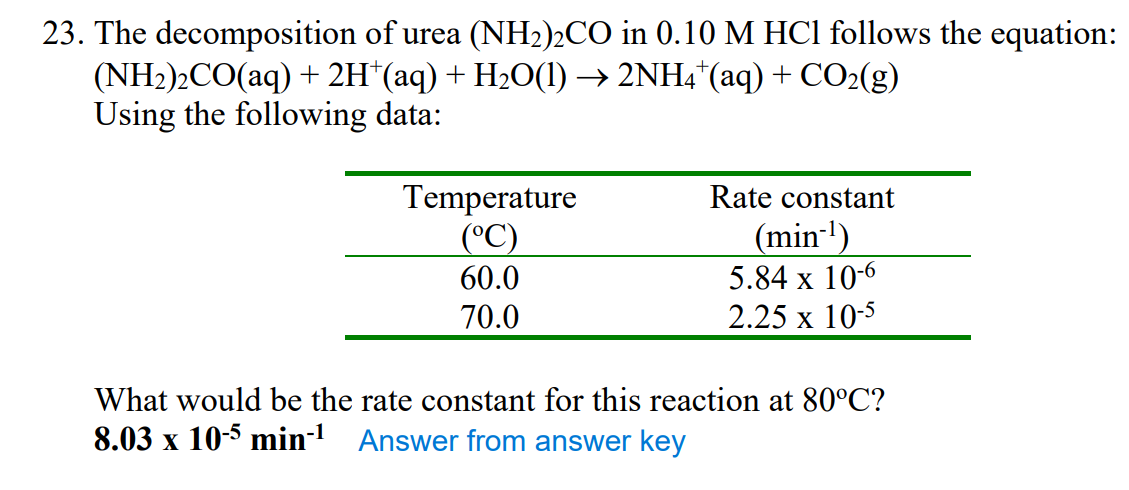
With increase in temperature, value of rate constant will also increse. So at higher temperature rate value will be highest.