Home /
Expert Answers /
Chemistry /
3-eyring-equation-an-un-catalyzed-reaction-sp-has-a-gibb-39-s-free-energy-of-activation-of-100-pa385
(Solved): 3. Eyring Equation. An un-catalyzed reaction (SP) has a Gibb's free energy of activation of +100 ...
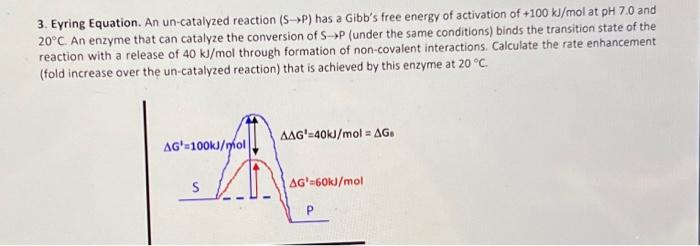
3. Eyring Equation. An un-catalyzed reaction has a Gibb's free energy of activation of at and . An enzyme that can catalyze the conversion of (under the same conditions) binds the transition state of the reaction with a release of through formation of non-covalent interactions. Calculate the rate enhancement (fold increase over the un-catalyzed reaction) that is achieved by this enzyme at .