Home /
Expert Answers /
Chemical Engineering /
3-consider-the-piston-cylinder-assembly-shown-below-it-is-well-insulated-and-initially-contains-t-pa742
(Solved): 3. Consider the piston cylinder assembly shown below. It is well insulated and initially contains t ...
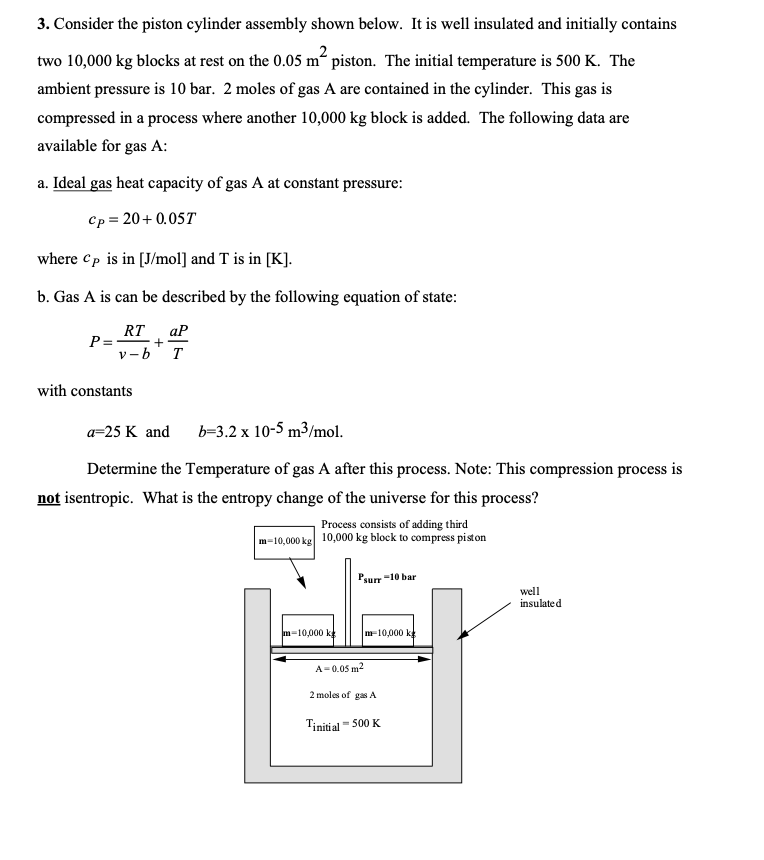
3. Consider the piston cylinder assembly shown below. It is well insulated and initially contains two blocks at rest on the piston. The initial temperature is . The ambient pressure is 10 bar. 2 moles of gas are contained in the cylinder. This gas is compressed in a process where another block is added. The following data are available for gas A: a. Ideal gas heat capacity of gas at constant pressure: where is in and is in . b. Gas A is can be described by the following equation of state: with constants Determine the Temperature of gas A after this process. Note: This compression process is not isentropic. What is the entropy change of the universe for this process?