Home /
Expert Answers /
Chemistry /
2pts-kinetics-of-an-lodine-clock-reaction-how-wal-you-collect-data-for-this-experiment-report-sh-pa711
(Solved): (2pts) Kinetics of an lodine-clock Reaction How wal you collect data for this experiment? Report Sh ...
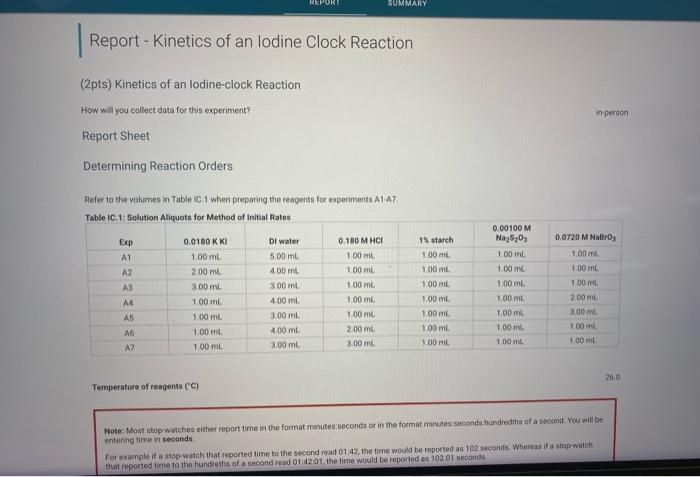
(2pts) Kinetics of an lodine-clock Reaction How wal you collect data for this experiment? Report Sheet Determining Reaction Orders Refer to the volumes in Table iC.1 when preparing the reagents for experiments A1.A7. Temperature of reagents ("C) Note- Most stop-watches eithes report time in the format minutes seconds or in the format minutes seconda hundrediths of a stcond. You will be sntening time in seconds For example if a stop-watch that roported time to the second iead 01 . 42, the time would be reported as 102 acconds. Whereas if a atop-watch that reported time to the hundleths of a second read 01 , the time would be reponted as seconds
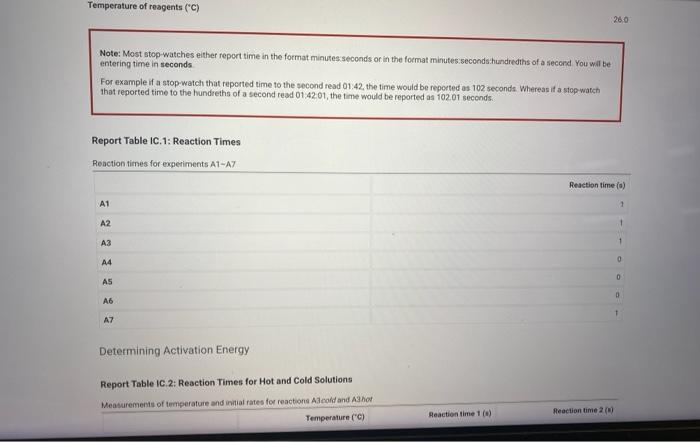
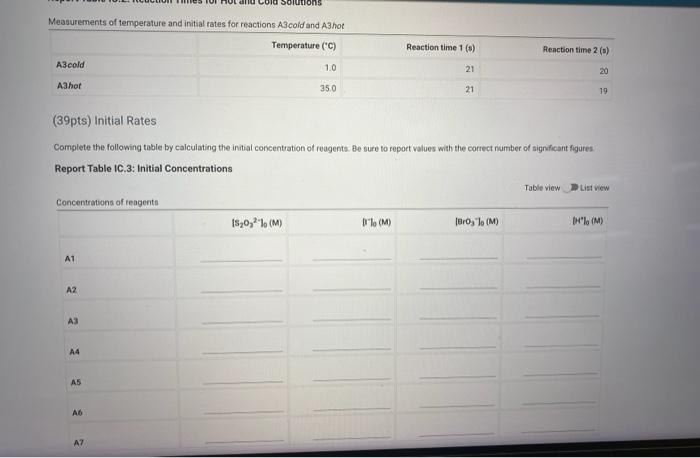
Note: Most stop-watches either report time in the format minuses seconds or in the format minutes.seconds hundredihs of a second. You wif be entering time in seconds. For example if a stop-watch that reported time to the second read , the time would be reported as 102 seconds Whereas if a stop watch that reported time to the hundreths of a second read 01.42.01, the time would be reported as seconds. Renort Table IC.1: Reaction Timne Determining Activation Energy Report Table IC. 2: Reaction Times for Hot and Cold Solutions Measurements of temperature and initial rates for reactions A Acodd and A3hot
(39pts) Initial Rates Complete the following table by calculating the initial concentration of reagents. Be sure to report values wish the correct number of significant figures. Report Table IC.3: Initial Concentrations
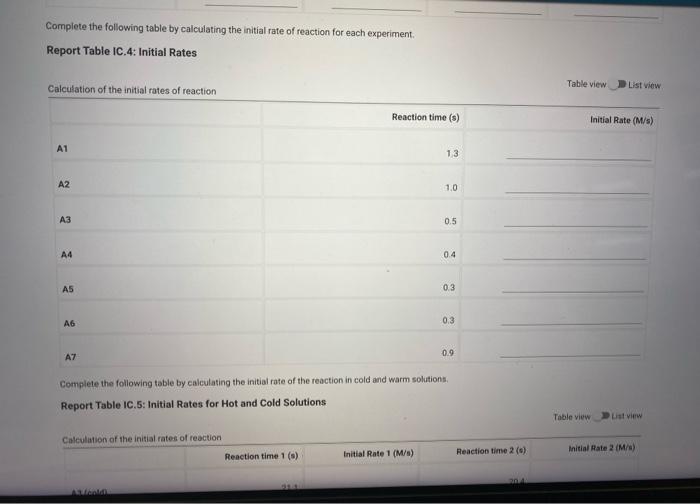
Complete the following table by calculating the initial rate of reaction for each experiment. Report Table IC.4: Initial Rates
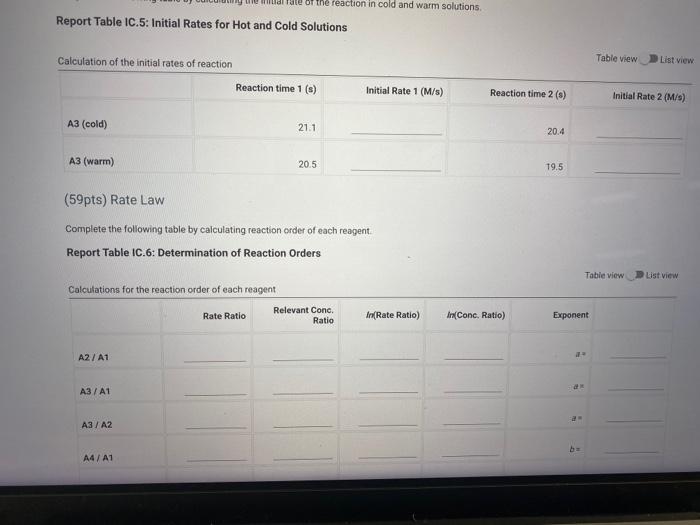
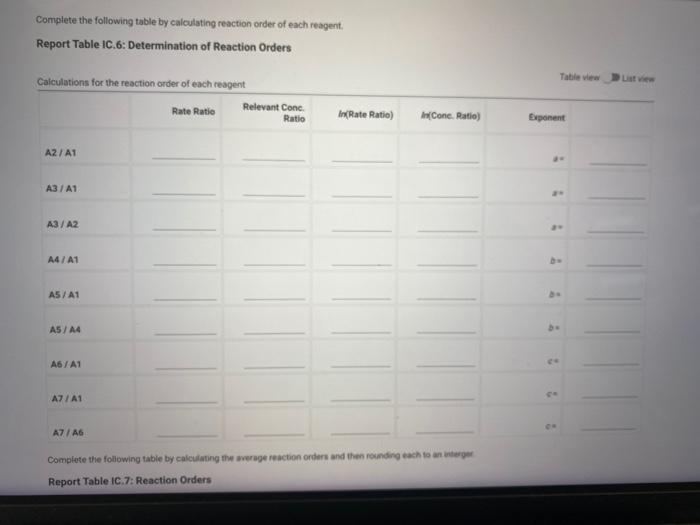
Report Table IC.5: Initial Rates for Hot and Cold Solutions Calculation of the initial rates of reaction Table view List view (59pts) Rate Law Complete the following table by calculating reaction order of each reagent. Report Table IC.6: Determination of Reaction Orders Table view R List view
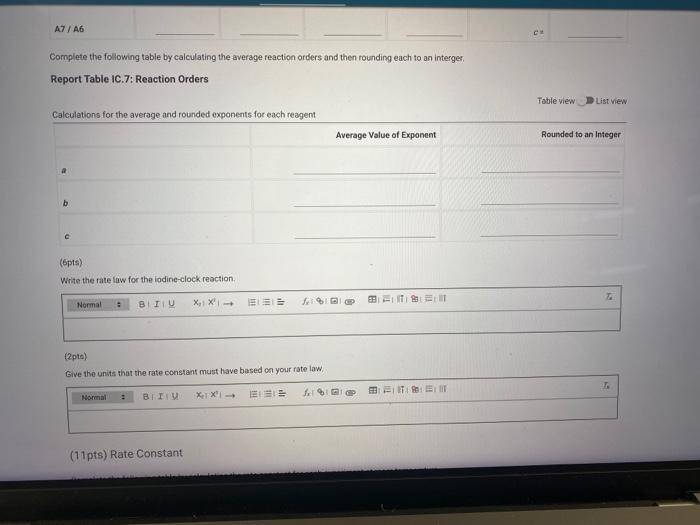
Complete the following table by calculating reaction order of each reagent. Report Table IC.6: Determination of Reaction Orders Calculations for the reaction order of each reagent Complote the following table by calculating the average reaction orders and then rowndry each to an wuengn Report Table IC.7: Reaction Orders
Complete the following table by calculating the average reaction orders and then rounding each to an interger. Report Table IC.7: Reaction Orders Ralewalatione fint thn aubrane and rouindewt avnonente for aach raanont (6pts)
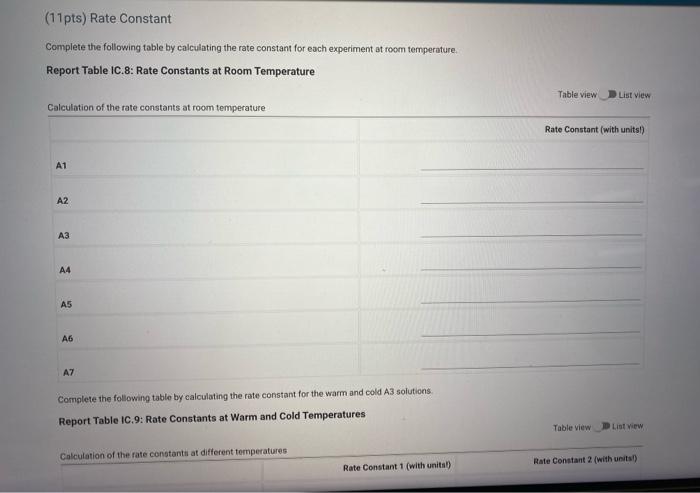
Complete the following table by calculating the rate constant for each experiment at room temperature. Report Table IC.8: Rate Constants at Room Temperature
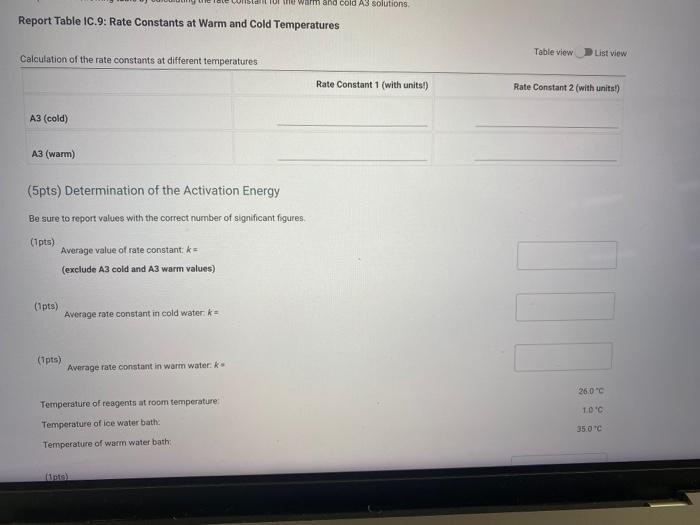
Report Table IC.9: Rate Constants at Warm and Cold Temperatures Calculation of the rate constants at different temperatures Tableviow Listiview Rate Constant 1 (with unitsl) Rate Constant 2 (with units?) (cold) A3 (warm) (5pts) Determination of the Activation Energy Be sure to report values with the correct number of significant figures. (1pts) Average value of rate constant: (exclude A3 cold and A3 warm values) (1pts) Average fate constant in cold water (ipts) Average rate corstant in warm water: Temperature of reagents at room temperature: Temperature of ice water bath: Temporature of warm water bath: (10is)
(Opto) Optional. Upload your spreadsheet, an tsx file; where you have done your reaction order and rate-constant calculations
Expert Answer
AnswerThe reaction between KI and NaBrO3 in presence of mineral acid is as follows:BrO3^- (aq) + 6 I- (aq) + 6 H+ (aq)13I2 (aq) + Br- (aq) + 3 H2O (l)