Home /
Expert Answers /
Chemistry /
25-consider-the-reaction-of-solid-p4-and-chlorine-gas-to-form-gaseous-phosphorus-trichloride-a-pa411
(Solved): 25 Consider the reaction of solid P4 and chlorine gas to form gaseous phosphorus trichloride. a += ...
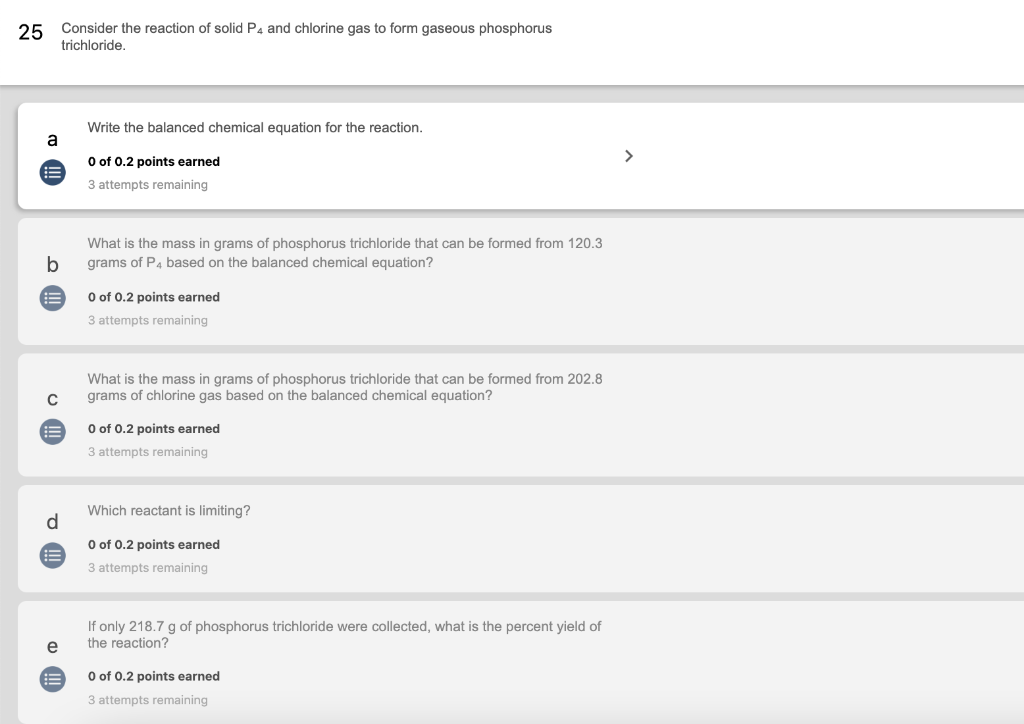
25 Consider the reaction of solid P4 and chlorine gas to form gaseous phosphorus trichloride. a += b == C d = e = Write the balanced chemical equation for the reaction. 0 of 0.2 points earned 3 attempts remaining What is the mass in grams of phosphorus trichloride that can be formed from 120.3 grams of P4 based on the balanced chemical equation? 0 of 0.2 points earned 3 attempts remaining What is the mass in grams of phosphorus trichloride that can be formed from 202.8 grams of chlorine gas based on the balanced chemical equation? 0 of 0.2 points earned 3 attempts remaining Which reactant is limiting? 0 of 0.2 points earned 3 attempts remaining If only 218.7 g of phosphorus trichloride were collected, what is the percent yield of the reaction? 0 of 0.2 points earned 3 attempts remaining
Expert Answer
Solution: Reaction Involved: Part A: Balancing the equation: Method Used: Heat and Trial. Step 1:Write the unbalanced chemical Equation: Step 2:Make the RHS with 4 Phosphoro