Home /
Expert Answers /
Chemistry /
22-a-major-line-in-the-emission-spectrum-of-an-unknown-element-corresponds-to-a-frequency-of-1-pa166
(Solved): 22. A major line in the emission spectrum of an unknown element corresponds to a frequency of \( 1. ...
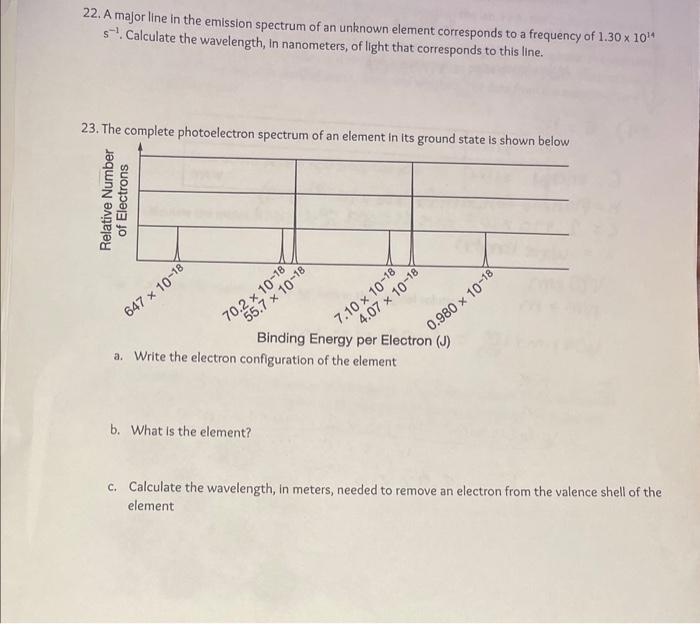
22. A major line in the emission spectrum of an unknown element corresponds to a frequency of \( 1.30 \times 10^{14} \) \( s^{-1} \). Calculate the wavelength, In nanometers, of light that corresponds to this line. 23. The complete photoelectron spectrum of an element in its ornund etato ie ehrum hal i... Binding Energy per Electron \( (\mathrm{J}) \) a. Write the electron configuration of the element b. What is the element? c. Calculate the wavelength, in meters, needed to remove an electron from the valence shell of the element