Home /
Expert Answers /
Chemistry /
20-question-1-point-use-the-data-below-to-calculate-delta-g-deg-rxn-for-the-reaction-a-2b-pa580
(Solved): 20 Question _((1 point )) Use the data below to calculate \Delta G\deg _(rxn) for the reaction: A+2B ...
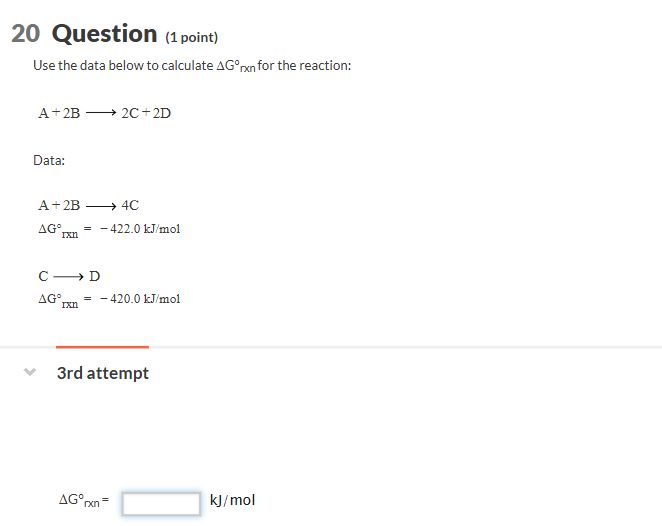
20 Question _((1 point ))
Use the data below to calculate \Delta G\deg _(rxn) for the reaction:
A+2Blongrightarrow2C+2D
Data:
A+2Blongrightarrow4C
\Delta G_(rxn)\deg =-422.0k(J)/(m)ol
ClongrightarrowD
\Delta G_(rxn)\deg =-420.0k(J)/(m)ol
3rd attempt
\Delta G_(rxn)\deg =,k(J)/(m)ol