Home /
Expert Answers /
Chemistry /
2-which-atom-is-oxidized-and-which-is-reduced-show-by-assigning-oxidation-39-s-in-the-following-e-pa368
(Solved): 2) Which atom is oxidized and which is reduced (show by assigning oxidation #'s) in the following e ...
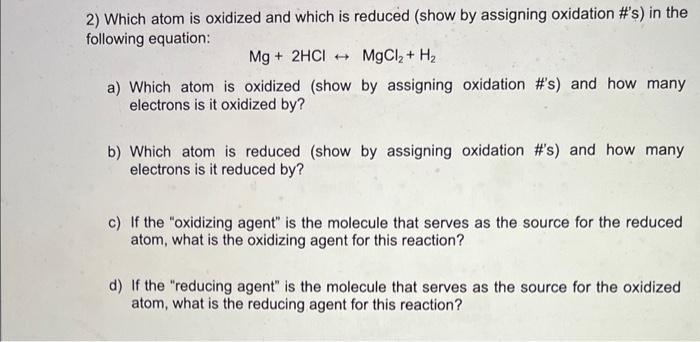
2) Which atom is oxidized and which is reduced (show by assigning oxidation #'s) in the following equation: \[ \mathrm{Mg}+2 \mathrm{HCl} \leftrightarrow \mathrm{MgCl}_{2}+\mathrm{H}_{2} \] a) Which atom is oxidized (show by assigning oxidation #'s) and how many electrons is it oxidized by? b) Which atom is reduced (show by assigning oxidation #'s) and how many electrons is it reduced by? c) If the "oxidizing agent" is the molecule that serves as the source for the reduced atom, what is the oxidizing agent for this reaction? d) If the "reducing agent" is the molecule that serves as the source for the oxidized atom, what is the reducing agent for this reaction?
Expert Answer
Answer: The given equation is as follows below. Mg + 2HCl MgCl2 + H2 (a) The atom which loses electrons is said to be oxidized. If the oxidation state of an atom increases then it undergoes oxidation. Let the oxidation state of magnesium (Mg) be a. O