Home /
Expert Answers /
Chemical Engineering /
2-triphenyl-methyl-ether-is-produced-from-the-reaction-of-triphenyl-methyl-chloride-with-methanol-pa929
(Solved): 2. Triphenyl methyl ether is produced from the reaction of triphenyl methyl chloride with methanol, ...
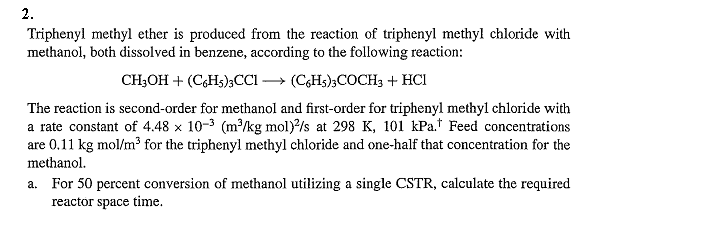
2. Triphenyl methyl ether is produced from the reaction of triphenyl methyl chloride with methanol, both dissolved in benzene, according to the following reaction: The reaction is second-order for methanol and first-order for triphenyl methyl chloride with a rate constant of at Feed concentrations are for the triphenyl methyl chloride and one-half that concentration for the methanol. a. For 50 percent conversion of methanol utilizing a single CSTR, calculate the required reactor space time.
Expert Answer
let's proceed to solve part (a) of the question, w...