Home /
Expert Answers /
Mechanical Engineering /
2-the-van-der-waals-equation-gives-a-relationship-between-the-pressure-p-in-atm-volume-v-in-l-pa464
(Solved): 2. The van der Waals equation gives a relationship between the pressure P (in atm.), volume V (in L ...
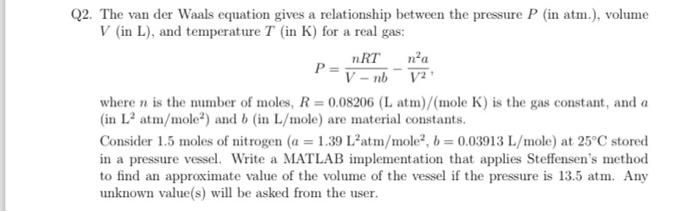
2. The van der Waals equation gives a relationship between the pressure (in atm.), volume (in ), and temperature (in ) for a real gas: where is the number of moles, mole is the gas constant, and (in ) and (in ) are material constants. Consider 1.5 moles of nitrogen at stored in a pressure vessel. Write a MATLAB implementation that applies Steffensen's method to find an approximate value of the volume of the vessel if the pressure is 13.5 atm. Any unknown value(s) will be asked from the user.