Home /
Expert Answers /
Chemical Engineering /
2-the-lead-tin-mathrm-pb-mathrm-sn-phase-diagram-is-shown-below-for-a-lead-tin-alloy-pa400
(Solved): 2. The lead-tin \( (\mathrm{Pb}-\mathrm{Sn}) \) phase diagram is shown below: For a lead-tin alloy ...
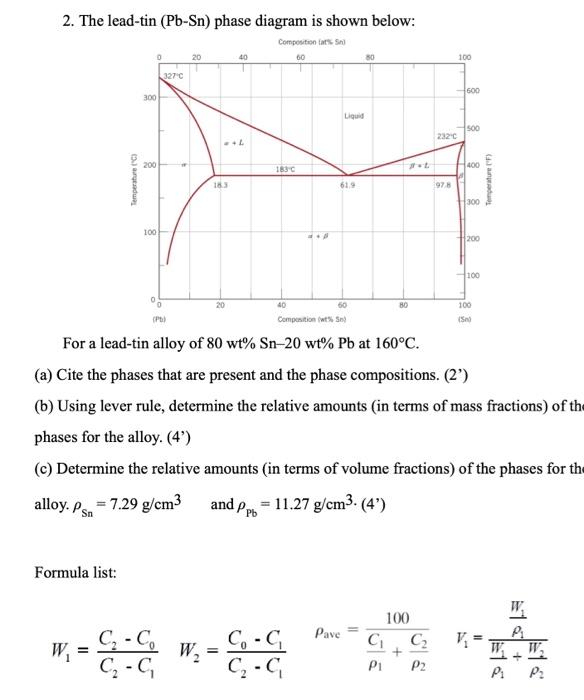
2. The lead-tin \( (\mathrm{Pb}-\mathrm{Sn}) \) phase diagram is shown below: For a lead-tin alloy of \( 80 \mathrm{wt} \% \mathrm{Sn}-20 \mathrm{wt} \% \mathrm{~Pb} \) at \( 160^{\circ} \mathrm{C} \). (a) Cite the phases that are present and the phase compositions. (2') (b) Using lever rule, determine the relative amounts (in terms of mass fractions) of th phases for the alloy. (4') (c) Determine the relative amounts (in terms of volume fractions) of the phases for th alloy. \( \rho_{\mathrm{Sn}}=7.29 \mathrm{~g} / \mathrm{cm}^{3} \quad \) and \( \rho_{\mathrm{Pb}}=11.27 \mathrm{~g} / \mathrm{cm}^{3} \cdot\left(4^{\prime}\right) \) Formula list: \[ W_{1}=\frac{C_{2}-C_{0}}{C_{2}-C_{1}} \quad W_{2}=\frac{C_{0}-C_{1}}{C_{2}-C_{1}} \quad \rho_{\text {ave }}=\frac{100}{\frac{C_{1}}{\rho_{1}}+\frac{C_{2}}{\rho_{2}}} \quad V_{1}=\frac{\frac{W_{1}}{\rho_{1}}}{\frac{W_{1}}{\rho_{1}}+\frac{W_{2}}{\rho_{2}}} \]