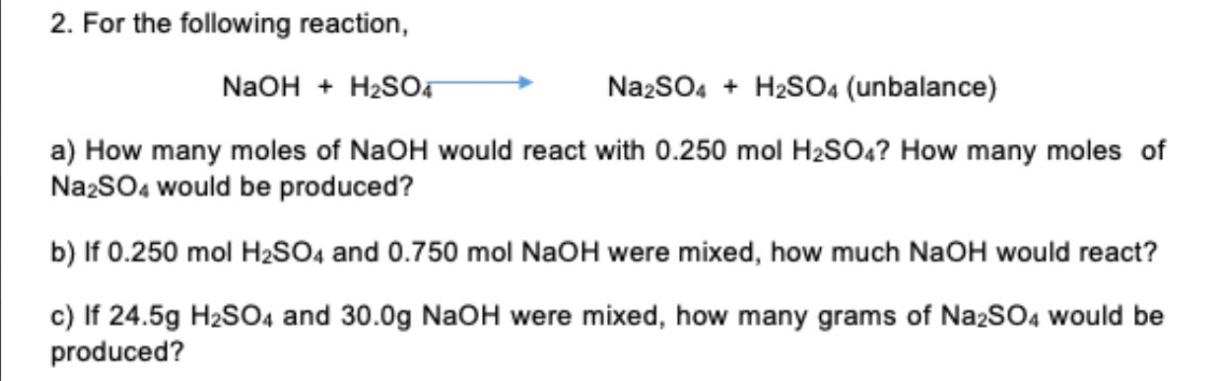Home /
Expert Answers /
Chemistry /
2-for-the-following-reaction-naoh-hso4-na2so4-h2so4-unbalance-a-how-many-moles-of-naoh-pa612
(Solved): 2. For the following reaction, NaOH + HSO4 Na2SO4 + H2SO4 (unbalance) a) How many moles of NaOH ...
2. For the following reaction, NaOH + H?SO4 Na2SO4 + H2SO4 (unbalance) a) How many moles of NaOH would react with 0.250 mol H?SO4? How many moles of Na2SO4 would be produced? b) If 0.250 mol H?SO4 and 0.750 mol NaOH were mixed, how much NaOH would react? c) If 24.5g H?SO4 and 30.0g NaOH were mixed, how many grams of Na2SO4 would be produced?
Expert Answer
The balanced chemical equation ; 2 NaOH + H2SO4 Na2SO4 + 2 H2O ; Q-2-a; From balanced equation 1 mole of H2SO4 gives rise to 1 mole of Na2SO4 ; 0.25 moles of H2SO4 how many moles of Na2SO4 is pr