Home /
Expert Answers /
Chemistry /
2-calculate-the-standard-enthalpy-of-formation-of-solid-magnesium-hydroxide-given-the-following-pa337
(Solved): 2. Calculate the standard enthalpy of formation of solid magnesium hydroxide, given the following ...
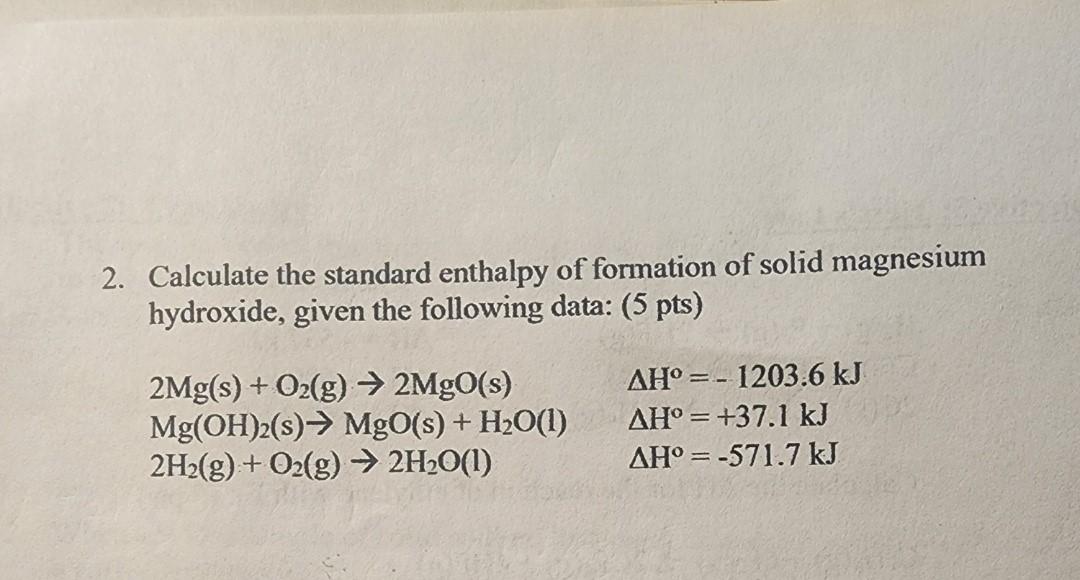
2. Calculate the standard enthalpy of formation of solid magnesium hydroxide, given the following data: (5 pts) \[ \begin{array}{ll} 2 \mathrm{Mg}(\mathrm{s})+\mathrm{O}_{2}(\mathrm{~g}) \rightarrow 2 \mathrm{MgO}(\mathrm{s}) & \Delta \mathrm{H}^{\mathrm{o}}=-1203.6 \mathrm{~kJ} \\ \mathrm{Mg}(\mathrm{OH})_{2}(\mathrm{~s}) \rightarrow \mathrm{MgO}(\mathrm{s})+\mathrm{H}_{2} \mathrm{O}(\mathrm{l}) & \Delta \mathrm{H}^{\mathrm{o}}=+37.1 \mathrm{~kJ} \\ 2 \mathrm{H}_{2}(\mathrm{~g})+\mathrm{O}_{2}(\mathrm{~g}) \rightarrow 2 \mathrm{H}_{2} \mathrm{O}(\mathrm{l}) & \Delta \mathrm{H}^{\mathrm{o}}=-571.7 \mathrm{~kJ} \end{array} \]