Home /
Expert Answers /
Chemistry /
2-calculate-the-molarity-of-the-crystal-violet-solutions-that-will-be-used-for-the-calibration-gr-pa873
(Solved): 2. Calculate the Molarity of the crystal violet solutions that will be used for the calibration gr ...
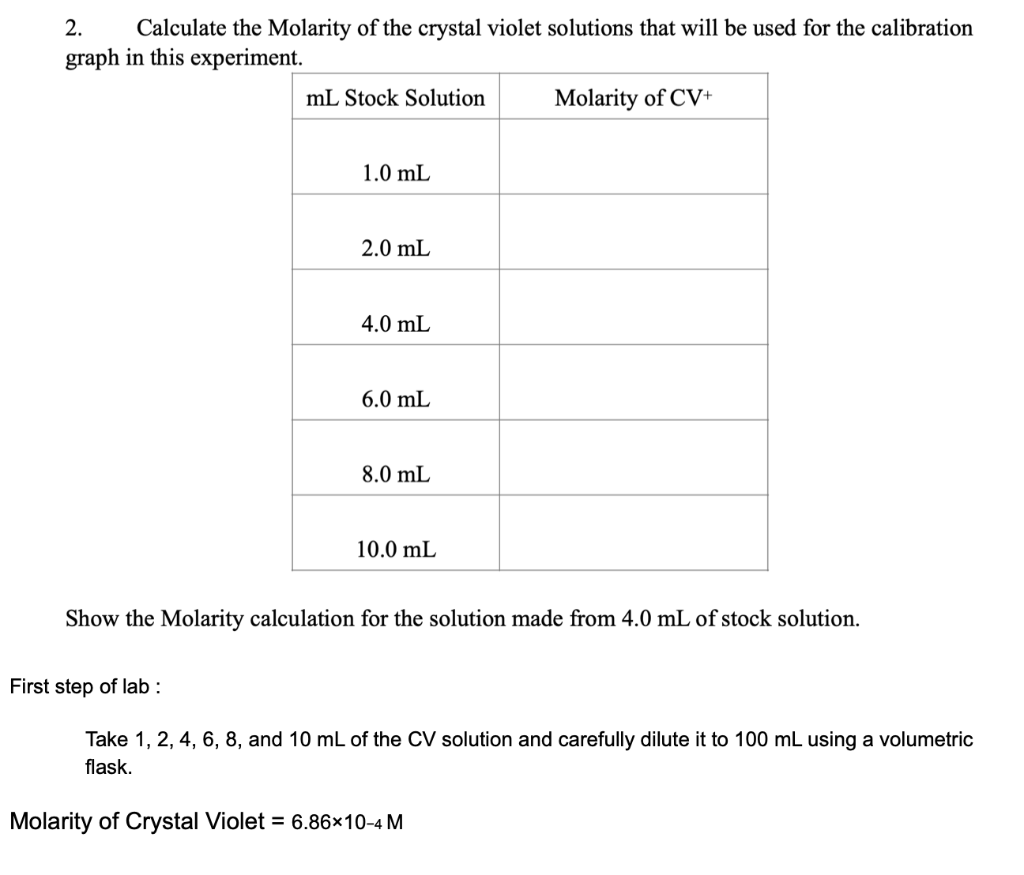
2. Calculate the Molarity of the crystal violet solutions that will be used for the calibration graph in this experiment. Show the Molarity calculation for the solution made from \( 4.0 \mathrm{~mL} \) of stock solution. irst step of lab : Take \( 1,2,4,6,8 \), and \( 10 \mathrm{~mL} \) of the \( \mathrm{CV} \) solution and carefully dilute it to \( 100 \mathrm{~mL} \) using a volumetric flask. Molarity of Crystal Violet \( =6.86 \times 10-4 \mathrm{M} \)
Expert Answer
Molarity of stock solution of crystal violet =6.86* 10-4 M= S1 1)Volume of the stock solution = 1 ml= V1 dilution is done upto = 100 ml= V2 Let the concentration of the final solution be = S2 We know that V1S1= V2S2 Now putting the values we get that