Home /
Expert Answers /
Chemistry /
2-calculate-formal-charges-on-the-mathrm-c-and-mathrm-o-atoms-in-two-resonance-str-pa359
(Solved): 2. Calculate formal charges on the \( \mathrm{C} \) and \( \mathrm{O} \) atoms in two resonance str ...
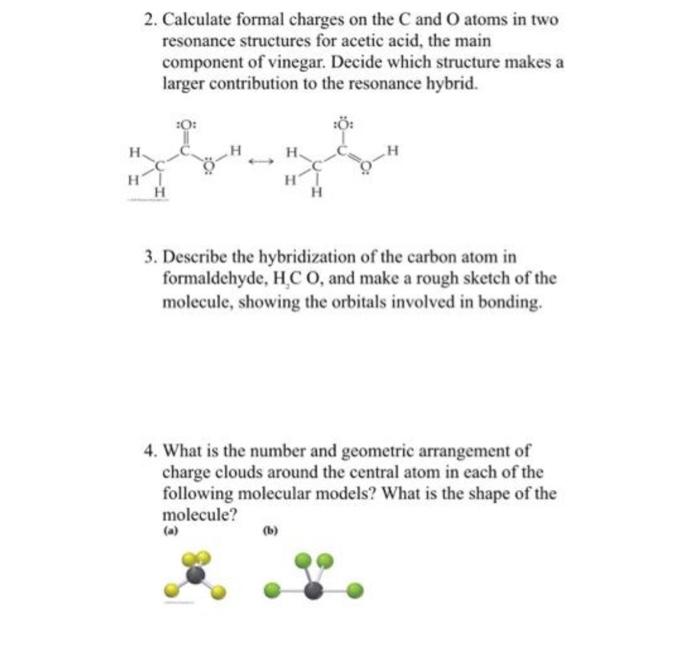
2. Calculate formal charges on the \( \mathrm{C} \) and \( \mathrm{O} \) atoms in two resonance structures for acetic acid, the main component of vinegar. Decide which structure makes a larger contribution to the resonance hybrid. 3. Describe the hybridization of the carbon atom in formaldehyde, \( \mathrm{H}_{2} \mathrm{C} \mathrm{O} \), and make a rough sketch of the molecule, showing the orbitals involved in bonding. 4. What is the number and geometric arrangement of charge clouds around the central atom in each of the following molecular models? What is the shape of the molecule?