Home /
Expert Answers /
Other Math /
2-a-bimolecular-reaction-in-which-a-and-b-combine-to-form-the-product-can-be-written-as-a-bkl-pa371
(Solved): 2. A bimolecular reaction in which A and B combine to form the product can be written as: A+Bkl ...
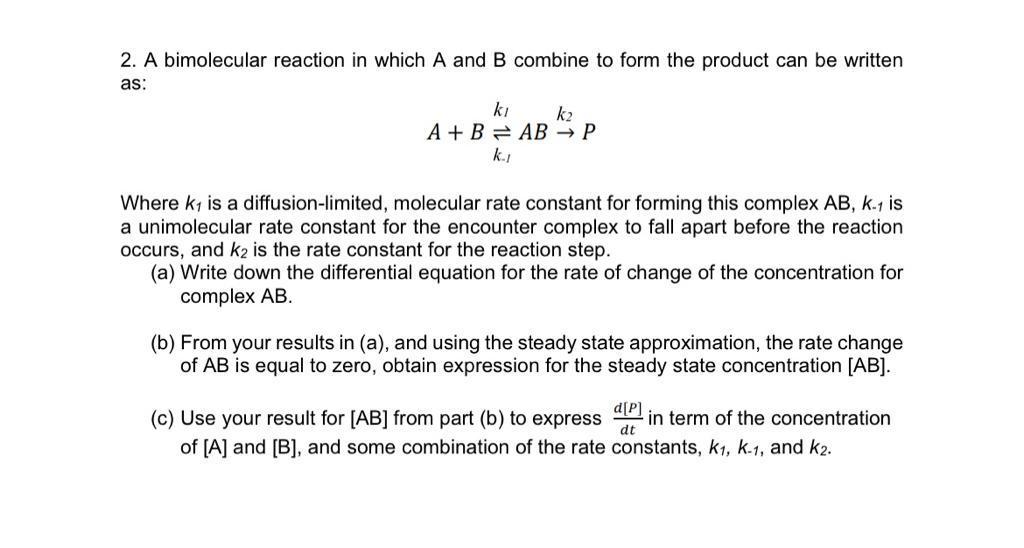
2. A bimolecular reaction in which and combine to form the product can be written as: Where is a diffusion-limited, molecular rate constant for forming this complex is a unimolecular rate constant for the encounter complex to fall apart before the reaction occurs, and is the rate constant for the reaction step. (a) Write down the differential equation for the rate of change of the concentration for complex AB. (b) From your results in (a), and using the steady state approximation, the rate change of is equal to zero, obtain expression for the steady state concentration . (c) Use your result for from part (b) to express in term of the concentration of and , and some combination of the rate constants, , and .