Home /
Expert Answers /
Chemistry /
19-15-marks-the-decomposition-of-hydrogen-peroxide-h2o2-was-studied-at-25c-the-react-pa747
(Solved): 19. (15 marks) The decomposition of hydrogen peroxide (H2O2) was studied at 25C. The react ...
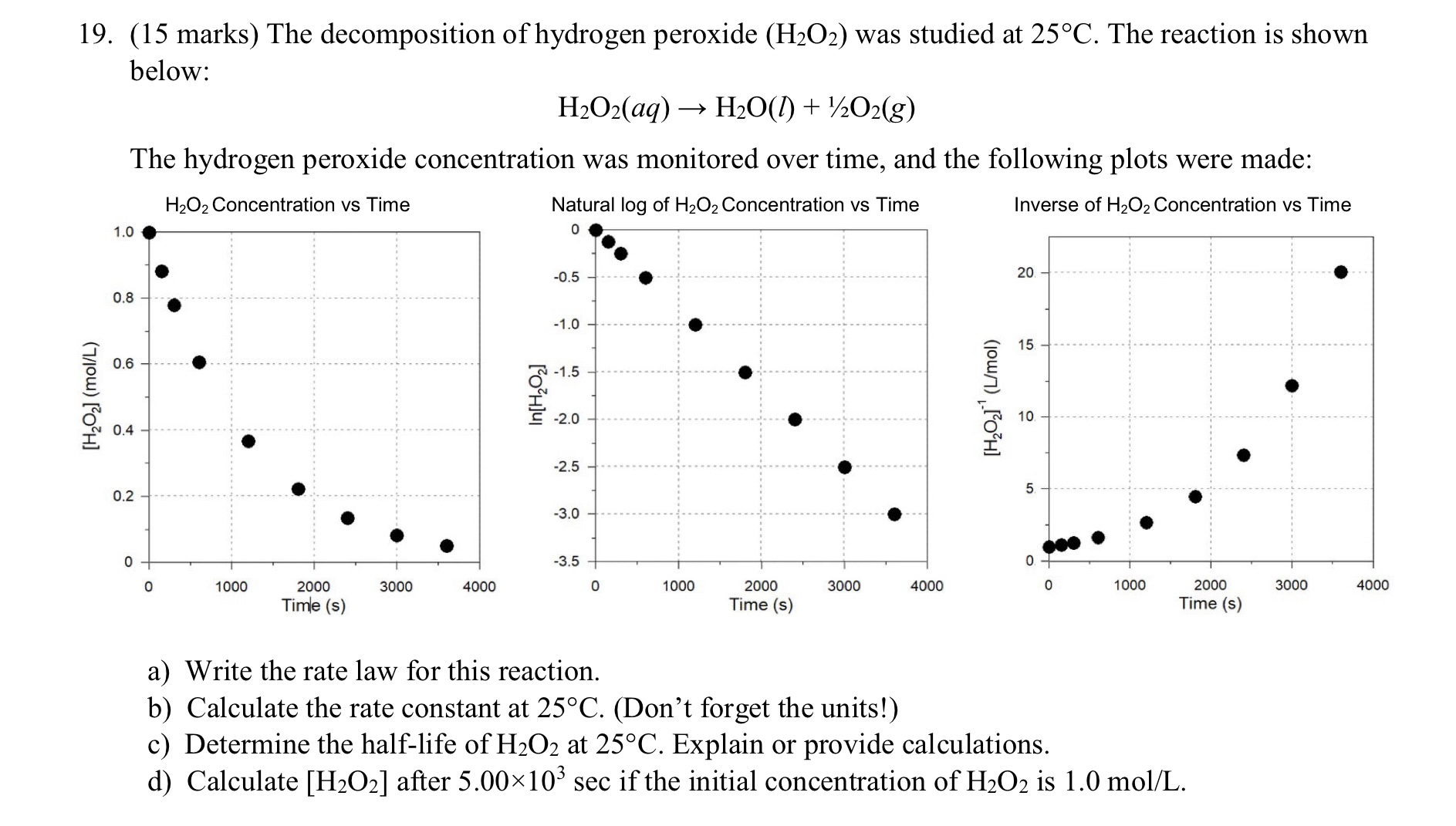
19. (15 marks) The decomposition of hydrogen peroxide was studied at . The reaction is shown below: The hydrogen peroxide concentration was monitored over time, and the following plots were made: a) Write the rate law for this reaction. b) Calculate the rate constant at . (Don't forget the units!) c) Determine the half-life of at . Explain or provide calculations. d) Calculate after if the initial concentration of is .
Expert Answer
let us consider the first order reaction and the integrated rate law formula for first order reaction is as follows where K is rate constant t is the time Co is initial concentration of the reactant andCt is the final concentration of the reactant .