Home /
Expert Answers /
Chemistry /
18-14-calculate-the-potential-of-a-zinc-electrode-immersed-in-a-0-0500-m-zn-no3-2-b-0-0200-m-i-pa349
(Solved): 18-14. Calculate the potential of a zinc electrode immersed in (a) 0.0500 M Zn(NO3)2. (b) 0.0200 M i ...
18-14. Calculate the potential of a zinc electrode immersed in (a) 0.0500 M Zn(NO3)2. (b) 0.0200 M in NaOH and saturated with Zn(OH)2. (c) 0.0150 M in Zn(NH3)2+ and 0.350 M in NH3-B4 for Zn(NH3)42+ is 7.76 × 108. adgim uo (d) Hq gnilem a solution in which the molar analytical concen- tration of Zn(NO3)2 is 4.00 × 10-³, that for H?Y2 is 0.0550 M, and the pH is fixed at 9.00. *1
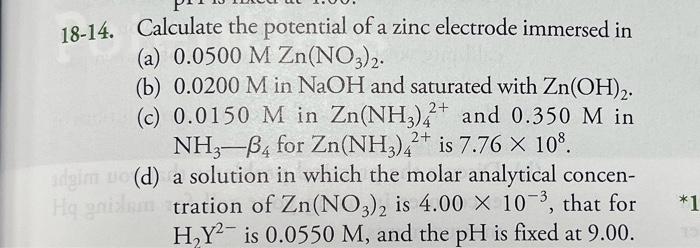
18-14. Calculate the potential of a zinc electrode immersed in (a) . (b) in and saturated with . (c) in and in for is . (d) a solution in which the molar analytical concentration of is , that for is , and the is fixed at 9.00 .
Expert Answer
(a) The standard reduction potential of is -0.76 V. The activity of in a 0.0500 M solution is 0.996. Therefore, the potential of the zinc electrode is:E = E° - * = -0.78 V(b) 0.0200 M in NaOH and saturated with The solubility product of is . The concentration of Zn2+ in a saturated solution of Zn(OH)2 is therefore M. The activity of Zn2+ in a 0.0200 M solution of NaOH is = . Therefore, the potential of the zinc electrode is:E = E° - 0.05916/2 * = -0.76 - 0.05916/2 * = -0.83 V