Home /
Expert Answers /
Chemistry /
17-neutron-activation-is-a-powerful-technique-that-can-be-used-for-useful-conversion-factors-ana-pa497
(Solved): 17. Neutron activation is a powerful technique that can be used for Useful conversion factors ana ...
17.
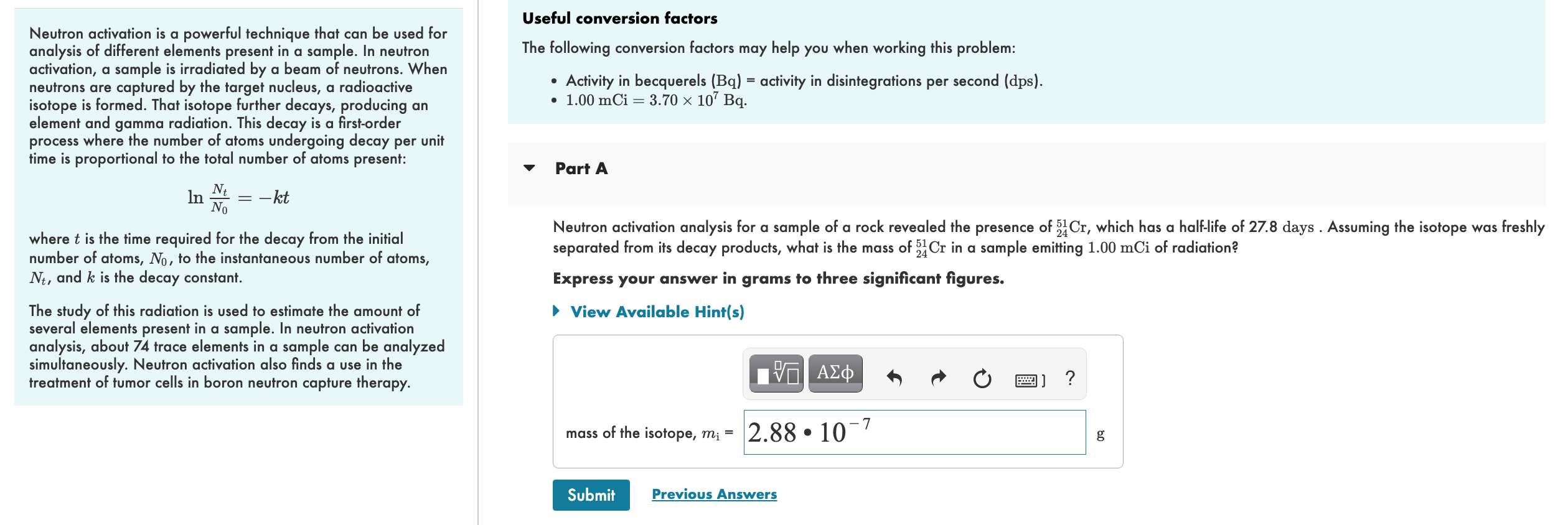
Neutron activation is a powerful technique that can be used for Useful conversion factors analysis of different elements present in a sample. In neutron The following conversion factors may help you when working this problem: activation, a sample is irradiated by a beam of neutrons. When neutrons are captured by the target nucleus, a radioactive - Activity in becquerels \( (\mathrm{Bq})= \) activity in disintegrations per second (dps). isotope is formed. That isotope further decays, producing an - \( 1.00 \mathrm{mCi}=3.70 \times 10^{7} \mathrm{~Bq} \). element and gamma radiation. This decay is a first-order process where the number of atoms undergoing decay per unit time is proportional to the total number of atoms present: \[ \ln \frac{N_{t}}{N_{0}}=-k t \] Part A where \( t \) is the time required for the decay from the initial Neutron activation analysis for a sample of a rock revealed the presence of \( { }_{24}^{51} \mathrm{Cr} \), which has a half-life of \( 27.8 \) days . Assuming the isotope was freshly number of atoms, \( N_{0} \), to the instantaneous number of atoms, separated from its decay products, what is the mass of \( { }_{24}^{51} \mathrm{Cr} \) in a sample emitting \( 1.00 \mathrm{mCi} \) of radiation? \( N_{t} \), and \( k \) is the decay constant. Express your answer in grams to three significant figures. The study of this radiation is used to estimate the amount of several elements present in a sample. In neutron activation analysis, about 74 trace elements in a sample can be analyzed simultaneously. Neutron activation also finds a use in the treatment of tumor cells in boron neutron capture therapy.
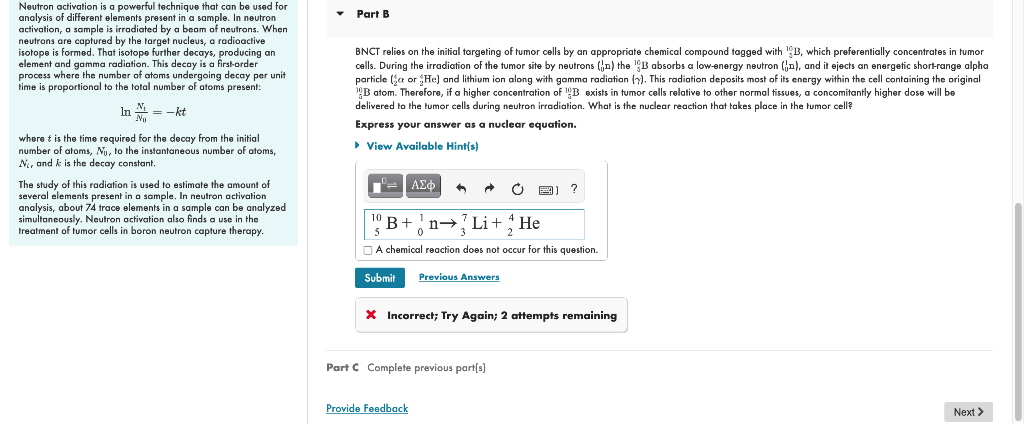
Neutron activation is a powerful technique that can be used for analysis of different elements present in a sample. In neutron activation, a sample is irradiated by a beam of neutrons. When neutrons are caplured by the larget nucleus, a radioactive isotope is formed. That isotope further decays, producing an element and gamma radiation. This decay is a first-order BNCT relies on the initial targeting of tumer cells by an appropriate chemical compound tagged with \( { }_{j}^{10} \mathrm{~B} \), which preferentially concentrates in tumer process where the number of atoms undergoing decay per unit cells. During the irradiation of the tumor site by neutrons \( \left({ }_{0}^{1} \mathrm{n}\right) \) the \( { }_{5}^{10} \mathrm{~B} \) absorbs a low-energy neutron \( \left({ }_{0}^{1} \mathrm{n}\right) \), and it ejects an energetic short-range alpha time is proportional to the total number of atoms present: particle \( \left({ }_{2}^{4} \alpha\right. \) or \( { }_{2}^{4} \mathrm{He} \) ) and lithium ion along with gamma radiation \( (\gamma) \). This radiation deposits most of its energy within the cell containing the original \( { }_{\mathrm{S}}^{10} \mathrm{~B} \) atom. Therefore, if a higher concentration of \( { }_{\mathrm{J}}^{10} \mathrm{~B} \) exists in tumor cells relative to other normal tissues, a concomitantly higher dose will be \[ \ln \frac{N_{1}}{N_{0}}=-k t \] delivered to the tumor cells during neutron irradiation. What is the nuclear reaction that takes place in the tumor cell? Express your answer as a nuclear equation. where \( t \) is the time required for the decay from the initial number of atoms, \( N_{0} \), to the instantaneous number of atoms, \( N_{i,} \), and \( k \) is the decay constant. The study of this radiation is used to estimate the amount of several elements present in a sample. In neulron activation analysis, about 74 trace elements in a sample can be analyzed simultaneously. Neutron activation also finds a use in the treatment of fumer cells in boron neutron capture therapy. X Incorrect; Try Again; 2 attempts remaining Part C Complete previous part[s]