Home /
Expert Answers /
Chemistry /
16-in-organic-chemistry-arrows-are-used-to-illustrate-the-flow-of-electrons-in-chemical-reactions-pa683
(Solved): 16. In organic chemistry, arrows are used to illustrate the flow of electrons in chemical reactions ...
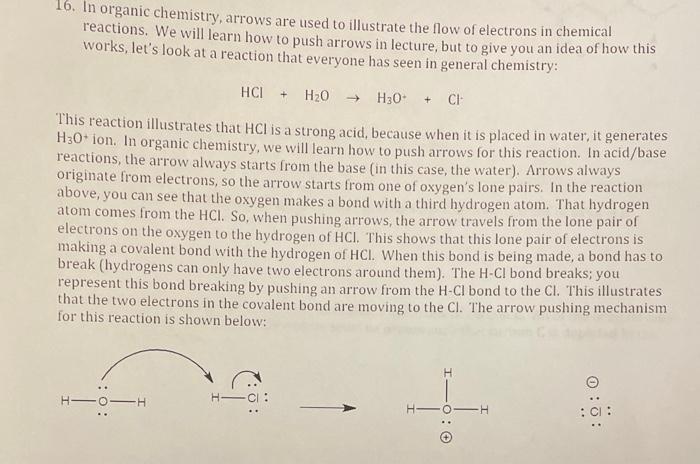
16. In organic chemistry, arrows are used to illustrate the flow of electrons in chemical reactions. We will learn how to push arrows in lecture, but to give you an idea of how this works, let's look at a reaction that everyone has seen in general chemistry: \[ \mathrm{HCl}+\mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{H}_{3} \mathrm{O}^{+}+\mathrm{Cl}^{+} \] This reaction illustrates that \( \mathrm{HCl} \) is a strong acid, because when it is placed in water, it generates \( \mathrm{H}_{3} \mathrm{O}^{+} \)ion. In organic chemistry, we will learn how to push arrows for this reaction. In acid/base reactions, the arrow always starts from the base (in this case, the water). Arrows always originate from electrons, so the arrow starts from one of oxygen's lone pairs. In the reaction above, you can see that the oxygen makes a bond with a third hydrogen atom. That hydrogen atom comes from the \( \mathrm{HCl} \). So, when pushing arrows, the arrow travels from the lone pair of electrons on the oxygen to the hydrogen of \( \mathrm{HCl} \). This shows that this lone pair of electrons is making a covalent bond with the hydrogen of \( \mathrm{HCl} \). When this bond is being made, a bond has to break (hydrogens can only have two electrons around them). The \( \mathrm{H}-\mathrm{Cl} \) bond breaks; you represent this bond breaking by pushing an arrow from the \( \mathrm{H}-\mathrm{Cl} \) bond to the \( \mathrm{Cl} \). This illustrates that the two electrons in the covalent bond are moving to the \( \mathrm{Cl} \). The arrow pushing mechanism for this reaction is shown below:
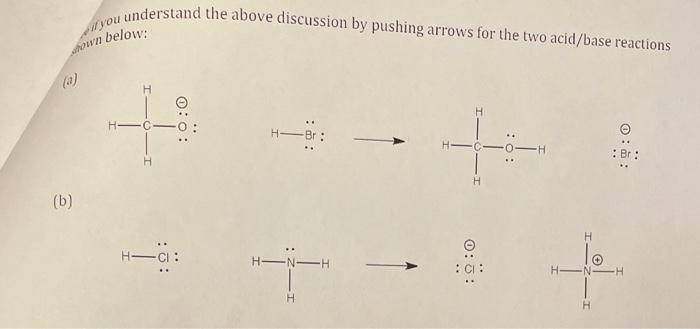
fown below: (a) (b)