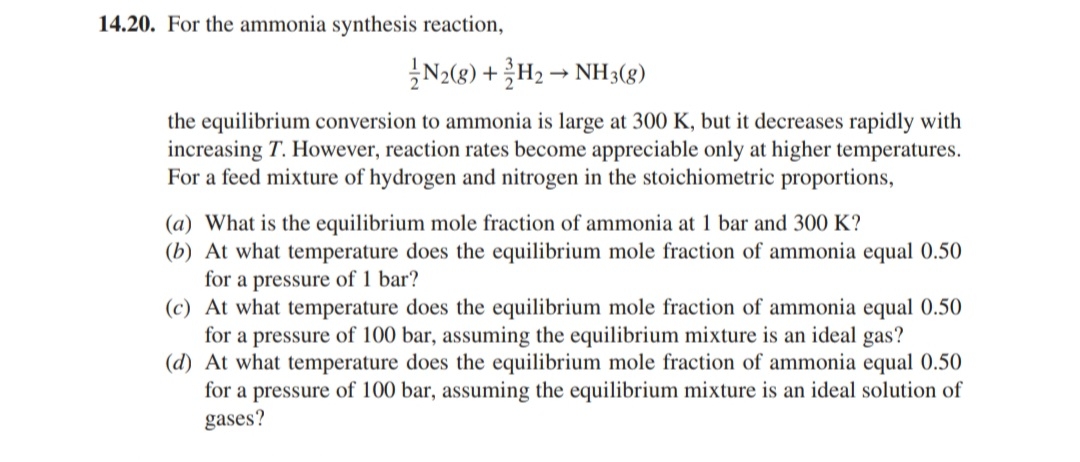
(Solved): 14.20. For the ammonia synthesis reaction, (1)/(2)N_(2)(g)+(3)/(2)H_(2)->NH_(3)(g) the equilibriu ...
14.20. For the ammonia synthesis reaction,
(1)/(2)N_(2)(g)+(3)/(2)H_(2)->NH_(3)(g)the equilibrium conversion to ammonia is large at 300 K , but it decreases rapidly with increasing
T. However, reaction rates become appreciable only at higher temperatures. For a feed mixture of hydrogen and nitrogen in the stoichiometric proportions, (a) What is the equilibrium mole fraction of ammonia at 1 bar and 300 K ? (b) At what temperature does the equilibrium mole fraction of ammonia equal 0.50 for a pressure of 1 bar? (c) At what temperature does the equilibrium mole fraction of ammonia equal 0.50 for a pressure of 100 bar , assuming the equilibrium mixture is an ideal gas? (d) At what temperature does the equilibrium mole fraction of ammonia equal 0.50 for a pressure of 100 bar , assuming the equilibrium mixture is an ideal solution of gases?