Home /
Expert Answers /
Chemistry /
13-calculate-the-heat-capacity-for-compound-y-for-all-three-trials-by-following-these-steps-pa483
(Solved): 13. Calculate the heat capacity for Compound \( Y \) for all three trials by following these steps ...
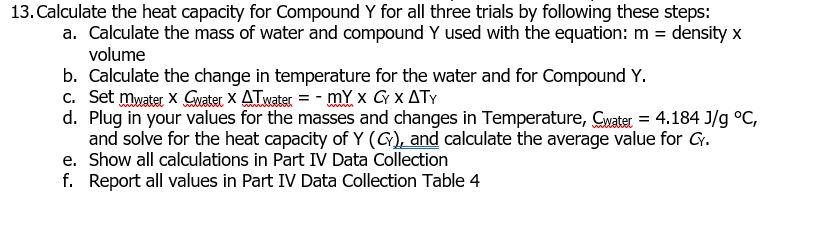
13. Calculate the heat capacity for Compound \( Y \) for all three trials by following these steps: a. Calculate the mass of water and compound \( Y \) used with the equation: \( m= \) density \( x \) volume b. Calculate the change in temperature for the water and for Compound \( Y \). c. Set \( m_{\text {watec }} \times C_{w a t e l} \times \Delta T_{w a t e r}=-m Y \times C_{Y} \times \Delta T_{Y} \) d. Plug in your values for the masses and changes in Temperature, \( C_{\text {water }}=4.184 \mathrm{~J} / \mathrm{g}^{\circ} \mathrm{C} \), and solve for the heat capacity of \( Y\left(C_{Y}\right) \), and calculate the average value for \( C_{Y} \). e. Show all calculations in Part IV Data Collection f. Report all values in Part IV Data Collection Table 4
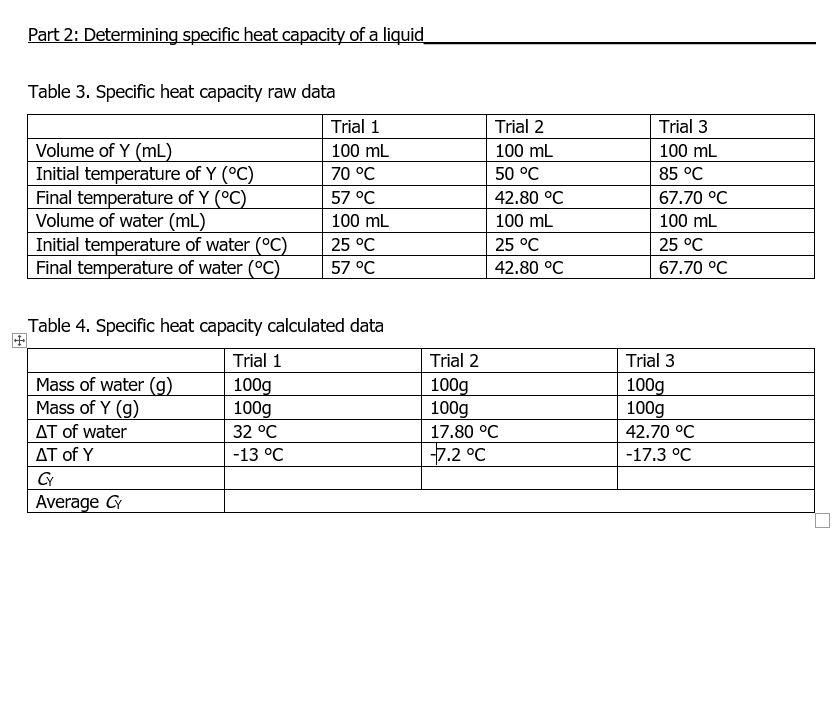
Part 2: Determining specific heat capacity of a liquid Table 3. Specific heat capacity raw data Table 4. Specific heat capacity calculated data
V. Applied Exercises Show all calculations necessary. Answer questions in COMPLETE sentences. 1. Is the heat capacity of Compound \( Y \) higher or lower than the heat capacity of ethylene glycol \( \left(2.200 \mathrm{~J} / \mathrm{g}^{\circ} \mathrm{C}\right) \) ? 2. Let's say you want to make a cappuccino, starting with \( 65^{\circ} \mathrm{C} \) frothed milk. How much frothed milk (in g) should you add to \( 200 \mathrm{~mL} \) of \( 95^{\circ} \mathrm{C} \) coffee to make a \( 90^{\circ} \mathrm{C} \) drink? Assume the density of coffee is equal to water \( (1.00 \mathrm{~g} / \mathrm{mL}) \). The specific heat of water is \( 4.184 \mathrm{~J} / \mathrm{g}{ }^{\circ} \mathrm{C} \) and the specific heat of milk is \( 3.890 \mathrm{~J} / \mathrm{g}{ }^{\circ} \mathrm{C} \).