Home /
Expert Answers /
Chemistry /
1234-what-is-the-frequency-of-light-if-its-wavelength-is-4-35-times-10-8-mathrm-m-pa150
(Solved): 1234 What is the frequency of light if its wavelength is \( 4.35 \times 10^{-8} \mathrm{~m} \) ? \( ...
1



2

3

4

What is the frequency of light if its wavelength is \( 4.35 \times 10^{-8} \mathrm{~m} \) ? \( 6.90 \times 10^{15} \mathrm{~s}^{-1} \) \( 1.93 \times 10^{15} \mathrm{~s}^{-1} \) \( 5.35 \times 10^{14} \mathrm{~s}^{-1} \) \( 6.70 \times 10^{15} s^{-1} \)
Of the sets of quantum numbers \( \left\{n, l, m_{\ell}\right. \), \( \left.m_{s}\right\} \), which is possible? \( \{3,1,0,1\} \) \( \{3,1,0,+1 / 2\} \) \( \{1,2,0,-1 / 2\} \) \( \{2,1,0,-1\} \)
Which of the following sets of quantum numbers \( \left\{n, \ell, m_{\ell}, m_{s}\right\} \) is a reasonable one for the atom oxygen at this ground state? \( \{3,1,0,1\} \) \( \{2,1,0,-1 / 2\} \) \( \{3,1,0,+1 / 2\} \) \( \{1,2,0,-1 / 2\} \)
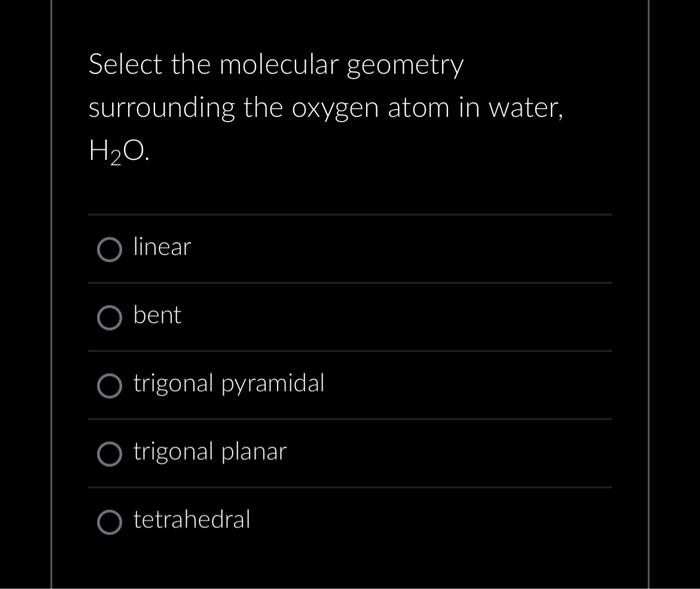
Select the molecular geometry surrounding the oxygen atom in water, \( \mathrm{H}_{2} \mathrm{O} \) linear bent trigonal pyramidal trigonal planar tetrahedral