Home /
Expert Answers /
Chemistry /
11-given-the-kinetic-energy-distribution-curves-and-threshold-energy-left-e-a-right-for-r-pa226
(Solved): 11.Given the kinetic energy distribution curves and threshold energy \( \left(E_{a}\right) \) for r ...
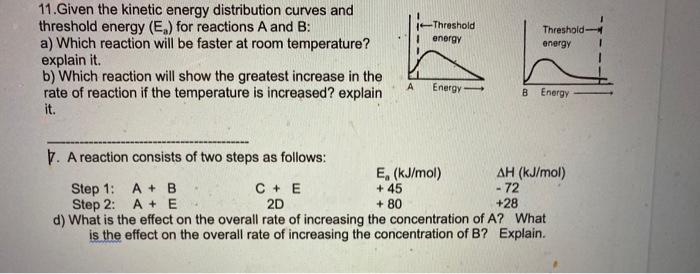
11.Given the kinetic energy distribution curves and threshold energy \( \left(E_{a}\right) \) for reactions \( A \) and \( B \) : a) Which reaction will be faster at room temperature? explain it. b) Which reaction will show the greatest increase in the rate of reaction if the temperature is increased? explain it. 7. A reaction consists of two steps as follows: Step 1: \( A+B \) Step 2: \( A+E \quad \) 2D \( \quad+80 \quad+28 \) d) What is the effect on the overall rate of increasing the concentration of \( A \) ? What