Home /
Expert Answers /
Chemistry /
10-do-the-following-calculations-for-a-10-00-mathrm-g-mathrm-mgnh-mathrm-m-pa306
(Solved): 10. \( { }^{*} \) Do the following calculations for a \( 10.00 \mathrm{~g} \mathrm{MgNH} \mathrm{M ...
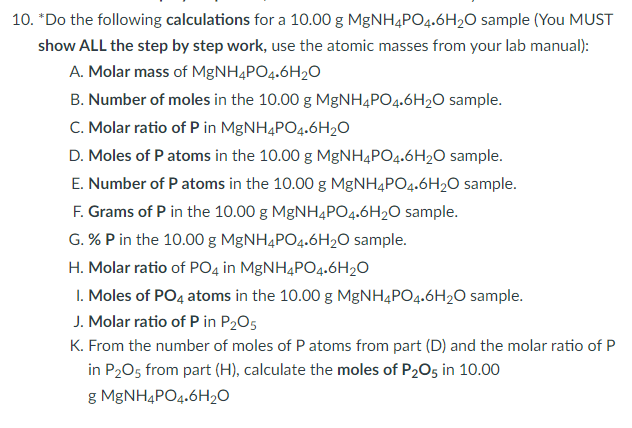
10. \( { }^{*} \) Do the following calculations for a \( 10.00 \mathrm{~g} \mathrm{MgNH} \mathrm{M}_{4} \mathrm{PO}_{4} \cdot 6 \mathrm{H}_{2} \mathrm{O} \) sample (You MUST show ALL the step by step work, use the atomic masses from your lab manual): A. Molar mass of \( \mathrm{MgNH}_{4} \mathrm{PO}_{4} \cdot 6 \mathrm{H}_{2} \mathrm{O} \) B. Number of moles in the \( 10.00 \mathrm{~g} \mathrm{MgNH} \mathrm{M}_{4} \mathrm{PO}_{4} \cdot 6 \mathrm{H}_{2} \mathrm{O} \) sample. C. Molar ratio of \( \mathrm{P} \) in \( \mathrm{MgNH}_{4} \mathrm{PO}_{4} \cdot 6 \mathrm{H}_{2} \mathrm{O} \) D. Moles of \( \mathrm{P} \) atoms in the \( 10.00 \mathrm{~g} \mathrm{MgNH} \mathrm{M}_{4} \mathrm{PO}_{4} \cdot 6 \mathrm{H}_{2} \mathrm{O} \) sample. E. Number of \( \mathrm{P} \) atoms in the \( 10.00 \mathrm{~g} \mathrm{MgNH}_{4} \mathrm{PO}_{4} .6 \mathrm{H}_{2} \mathrm{O} \) sample. F. Grams of \( \mathrm{P} \) in the \( 10.00 \mathrm{~g} \mathrm{MgNH} \mathrm{H}_{4} \mathrm{PO}_{4} \cdot 6 \mathrm{H}_{2} \mathrm{O} \) sample. G. \% P in the \( 10.00 \mathrm{~g} \mathrm{MgNH}_{4} \mathrm{PO}_{4} \cdot 6 \mathrm{H}_{2} \mathrm{O} \) sample. H. Molar ratio of \( \mathrm{PO}_{4} \) in \( \mathrm{MgNH}_{4} \mathrm{PO}_{4} \cdot 6 \mathrm{H}_{2} \mathrm{O} \) I. Moles of \( \mathrm{PO}_{4} \) atoms in the \( 10.00 \mathrm{~g} \mathrm{MgNH} \mathrm{MPO}_{4} \cdot 6 \mathrm{H}_{2} \mathrm{O} \) sample. J. Molar ratio of \( \mathrm{P} \) in \( \mathrm{P}_{2} \mathrm{O}_{5} \) K. From the number of moles of \( P \) atoms from part \( (D) \) and the molar ratio of \( P \) in \( \mathrm{P}_{2} \mathrm{O}_{5} \) from part \( (\mathrm{H}) \), calculate the moles of \( \mathrm{P}_{2} \mathrm{O}_{5} \) in \( 10.00 \) g \( \mathrm{MgNH}_{4} \mathrm{PO}_{4} \cdot 6 \mathrm{H}_{2} \mathrm{O} \)