Home /
Expert Answers /
Chemistry /
10-calculate-the-wavelength-of-the-light-emitted-by-a-hydrogen-atom-during-the-electronic-transiti-pa734
(Solved): 10. Calculate the wavelength of the light emitted by a hydrogen atom during the electronic transiti ...
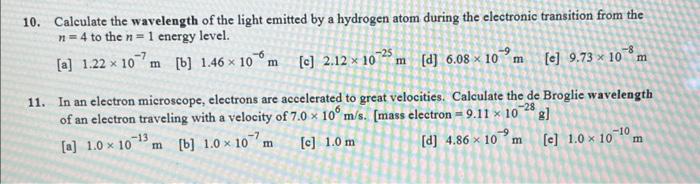
10. Calculate the wavelength of the light emitted by a hydrogen atom during the electronic transition from the to the energy level. [a] [b] [c] [d] [e] 11. In an electron microscope, electrons are accelerated to great velocities. Calculate the de Broglie wavelength of an electron traveling with a velocity of . [mass electron ] [a] [b] [c] [d] [c]