Home /
Expert Answers /
Chemistry /
1-when-the-following-molecular-equation-is-balanced-using-the-smallest-possible-integer-coef-pa747
(Solved): \( (1 \) When the following molecular equation is balanced using the smallest possible integer coef ...
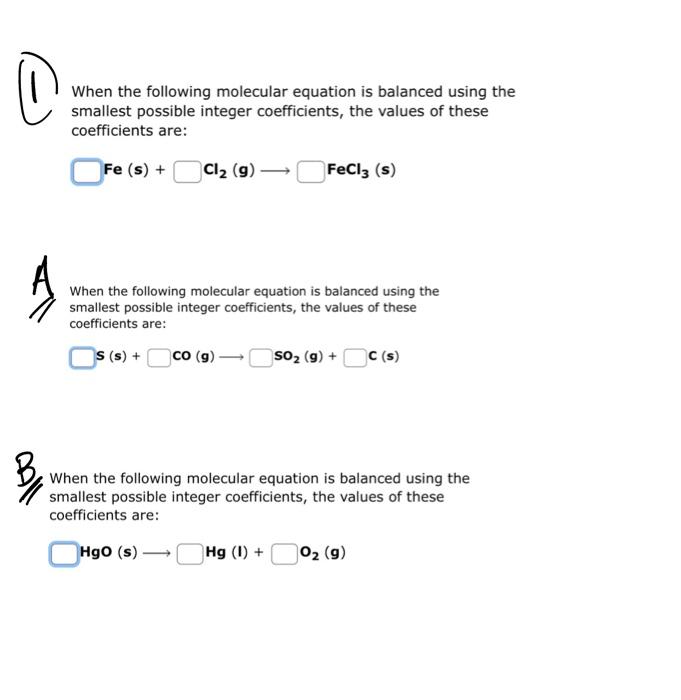
\( (1 \) When the following molecular equation is balanced using the smallest possible integer coefficients, the values of these coefficients are: \( \mathrm{Fe}(\mathrm{s})+\mathrm{Cl}_{2}(\mathrm{~g}) \longrightarrow \mathrm{FeCl}_{3}(\mathrm{~s}) \) When the following molecular equation is balanced using the smallest possible integer coefficients, the values of these coefficients are: \[ \mathbf{s}(\mathrm{s})+\quad \mathrm{CO}(\mathrm{g}) \longrightarrow \quad \mathrm{SO}_{2}(\mathrm{~g})+\quad \mathrm{C}(\mathrm{s}) \] 1. When the following molecular equation is balanced using the smallest possible integer coefficients, the values of these coefficients are: \[ \mathrm{HgO}(\mathrm{s}) \longrightarrow \quad \mathrm{Hg}(\mathrm{I})+\quad \mathrm{O}_{2}(\mathrm{~g}) \]