Home /
Expert Answers /
Mechanical Engineering /
1-two-phase-equilibrium-we-consider-a-system-with-two-phases-j-alpha-beta-having-the-mole-fracti-pa543
(Solved): 1 Two phase equilibrium We consider a system with two phases j=\alpha ,\beta having the mole fracti ...
1 Two phase equilibrium We consider a system with two phases
j=\alpha ,\beta having the mole fractions
n^(\alpha ),n^(\beta )where the total amount is
n_(A)+n_(B). Each component
i=A,Bin both phases can be described by a chemical potential
\mu _(i)^(j)and its corresponding mole fraction
n_(i)^(j). For the thermodynamic equilibrium the free enthalpy of the systems is
G=\sum_(i=A) ,B\sum_(j=\alpha ) ,\beta n_(i)^(j)\mu _(i)^(j)is minimal, with
n_(A)^(\alpha )+n_(A)^(\beta )=n_(A)=const. and
n_(B)^(\alpha )+n_(B)^(\beta )=n_(B)=const. Determine the minimum of the total free enthalpy
Gwith the constraint of mass conservation. Show that for thermodynamic equilibrium the chemical potentials are identical:
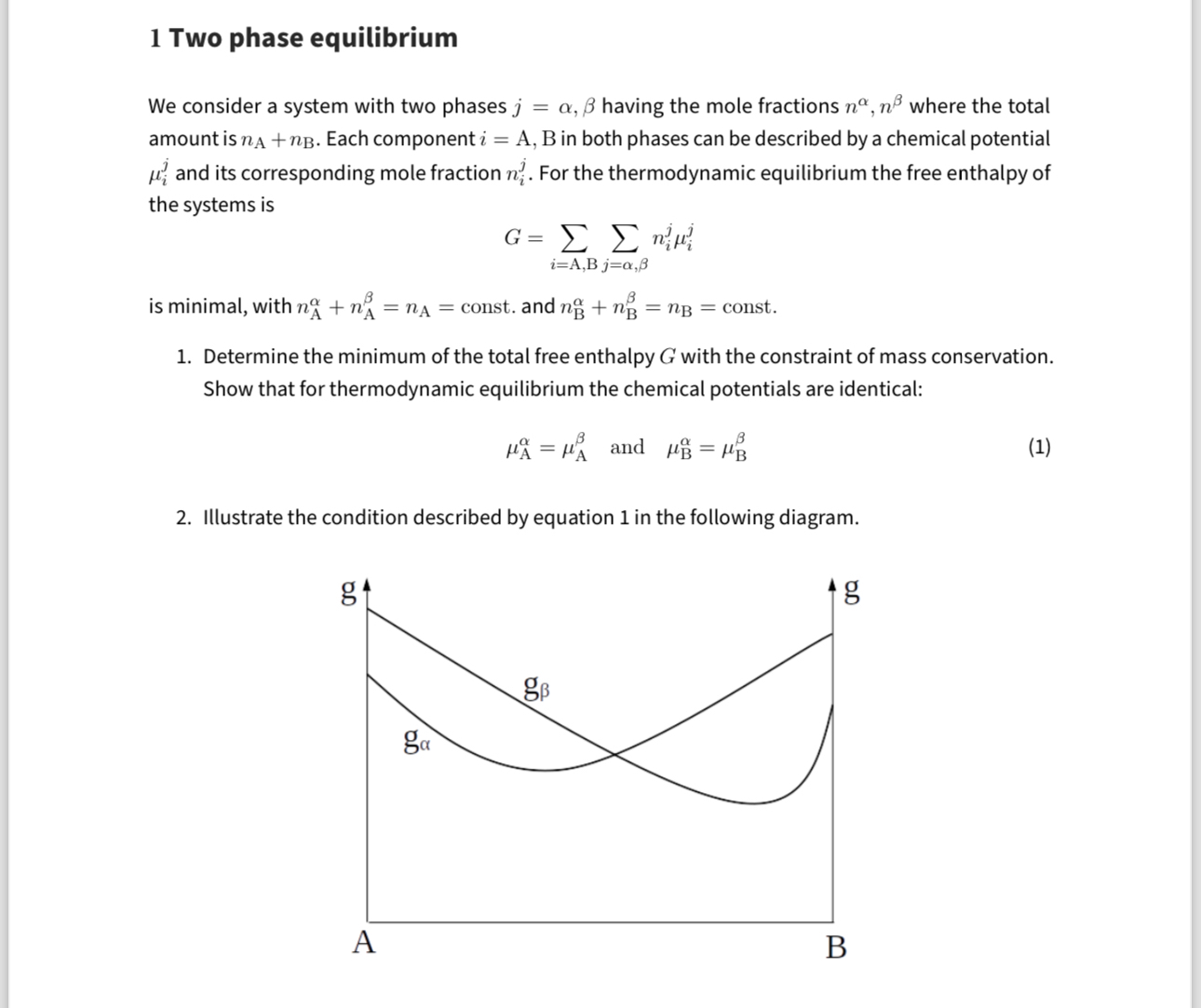
\mu _(A)^(\alpha )=\mu _(A)^(\beta ), and ,\mu _(B)^(\alpha )=\mu _(B)^(\beta )Illustrate the condition described by equation 1 in the following diagram.