Home /
Expert Answers /
Advanced Physics /
1-the-theoretical-specific-heat-capacity-of-a-solid-at-temperature-t-is-given-by-the-debye-formula-pa687
(Solved): 1. The theoretical specific heat capacity of a solid at temperature T is given by the Debye formula ...
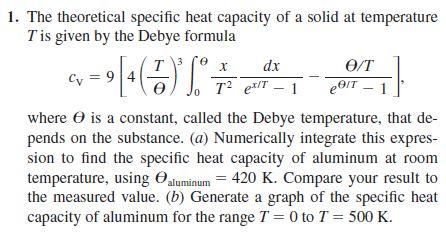
1. The theoretical specific heat capacity of a solid at temperature T is given by the Debye formula
where U is a constant, called the Debye temperature, that
depends
on the substance. (a) Numerically integrate this expression
to find the specific heat capacity of aluminum at room
temperature, using Ualuminum 420 K. Compare your result to
the measured value. (b) Generate a graph of the specific heat
capacity of aluminum for the range T 0 to T 500 K.
1. The theoretical specific heat capacity of a solid at temperature \( T \) is given by the Debye formula \[ c_{\mathrm{V}}=9\left[4\left(\frac{T}{\theta}\right)^{3} \int_{0}^{\theta} \frac{x}{T^{2}} \frac{d x}{e^{x / T}-1}-\frac{\theta / T}{e^{\theta / T}-1}\right] \] where \( \theta \) is a constant, called the Debye temperature, that depends on the substance. \( (a) \) Numerically integrate this expression to find the specific heat capacity of aluminum at room temperature, using \( \Theta_{\text {aluminum }}=420 \mathrm{~K} \). Compare your result to the measured value. (b) Generate a graph of the specific heat capacity of aluminum for the range \( T=0 \) to \( T=500 \mathrm{~K} \).
Expert Answer
Given formula for specific heat : which can be modified to: At high temperatures, (theta/T) and x both tends