Home /
Expert Answers /
Chemistry /
1-the-standard-enthalpy-of-formation-for-rhodium-dichromate-is-450kj-mol-write-the-thermochemic-pa564
(Solved): 1. The standard enthalpy of formation for rhodium dichromate is 450kJ/mol. Write the thermochemic ...
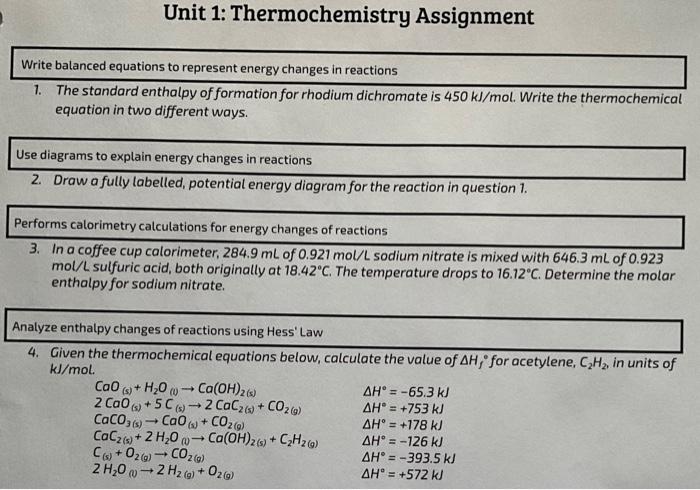
1. The standard enthalpy of formation for rhodium dichromate is . Write the thermochemical equation in two different ways. Use diagrams to explain energy changes in reactions 2. Draw a fully labelled, potential energy diagram for the reaction in question 1. Performs calorimetry calculations for energy changes of reactions 3. In a coffee cup calorimeter, of sodium nitrate is mixed with of mol/L sulfuric acid, both originally at . The temperature drops to . Determine the molar enthalpy for sodium nitrate. Analyze enthalpy changes of reactions using Hess' Law