Home /
Expert Answers /
Chemistry /
1-the-formula-for-the-conjugate-base-of-h2co3-is-2-the-formula-for-the-conjugate-acid-of-hs-pa434
(Solved): 1. The formula for the conjugate base of H2CO3 is 2. The formula for the conjugate acid of HS ...
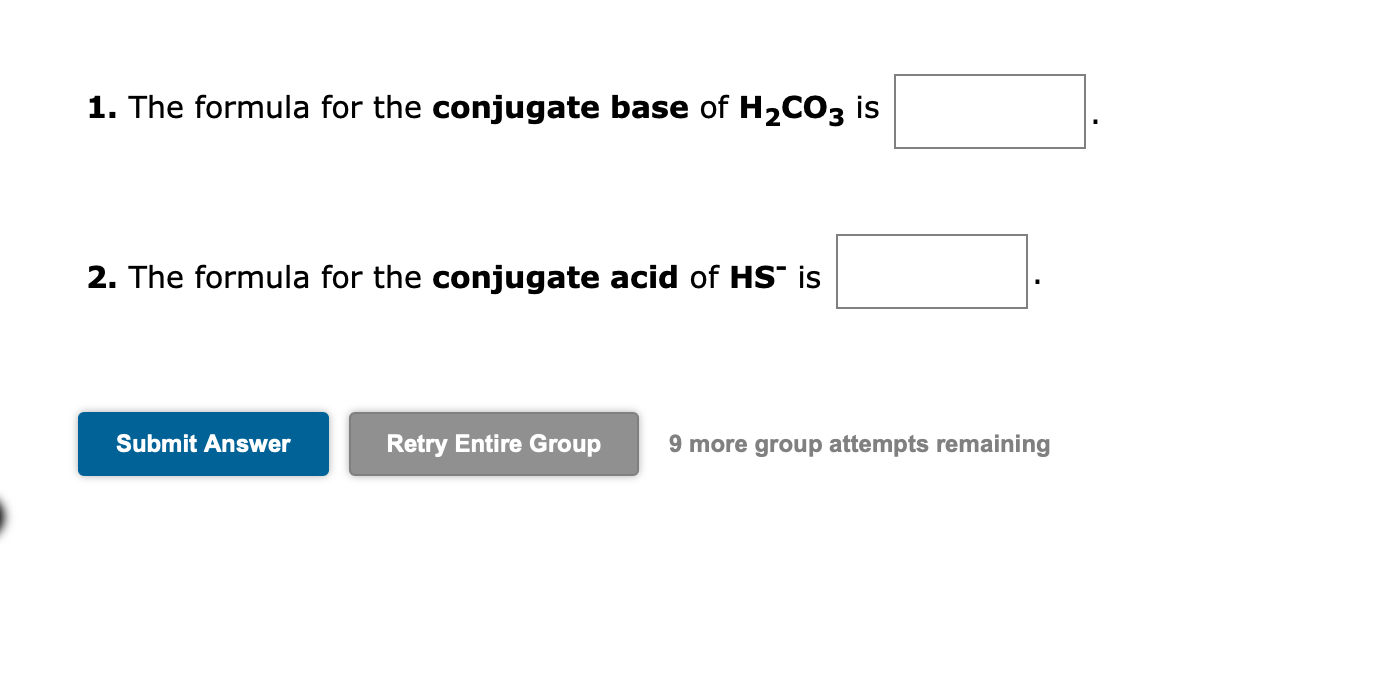
1. The formula for the conjugate base of is 2. The formula for the conjugate acid of is
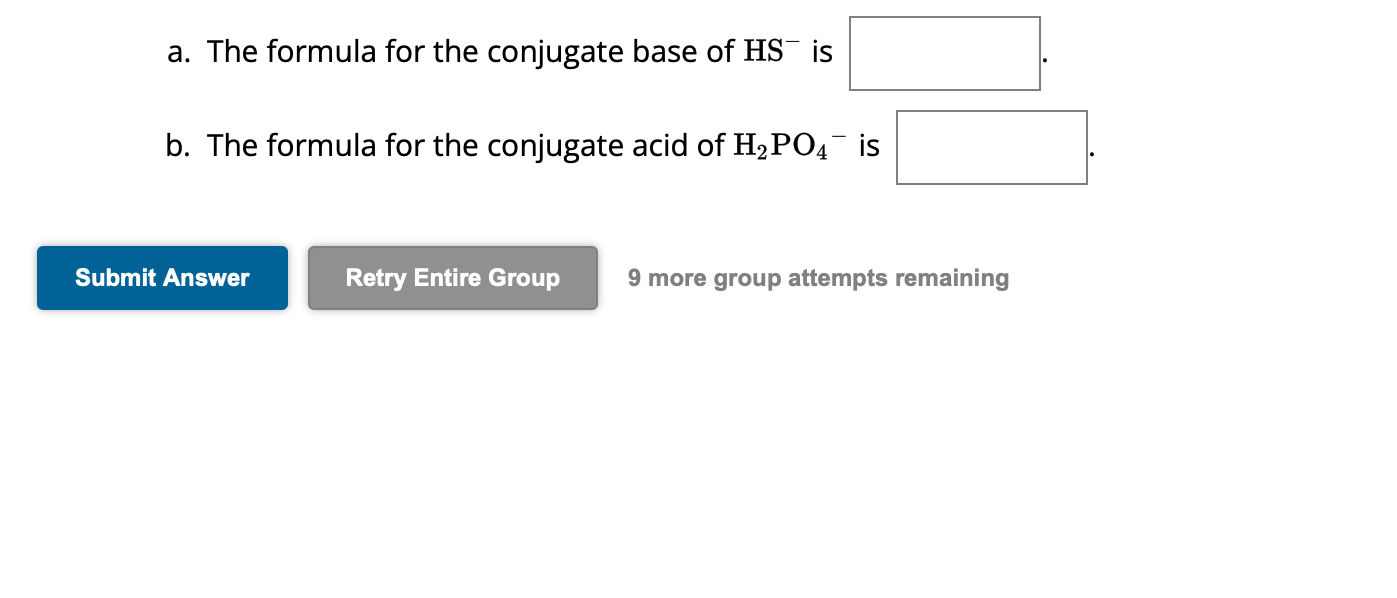
a. The formula for the conjugate base of is b. The formula for the conjugate acid of is 9 more group attempts remaining
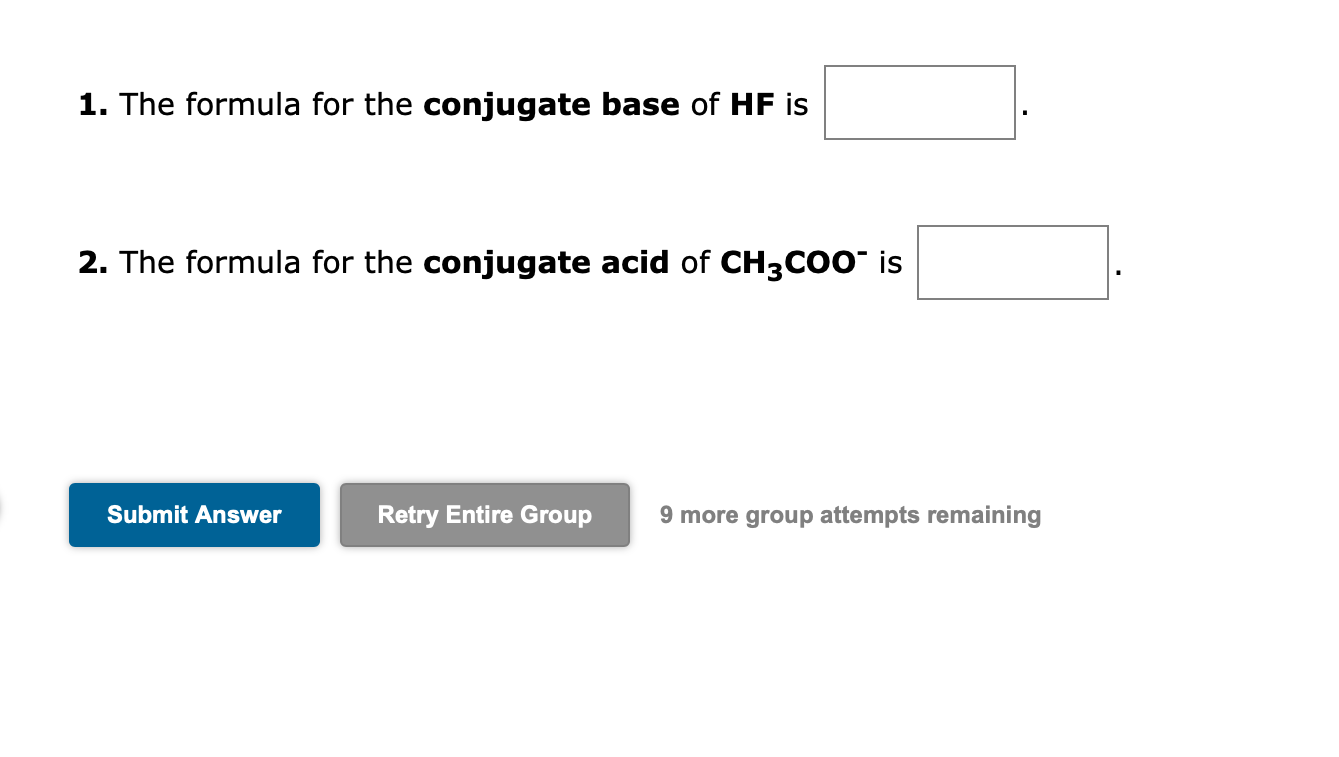
1. The formula for the conjugate base of HF is 2. The formula for the conjugate acid of is 9 more group attempts remaining
Expert Answer
Acid:- According to the Bronsted-Lowry concept acid is a substance which can release proton (H+).Base:-