Home /
Expert Answers /
Chemistry /
1-soluble-compounds-dissolve-in-water-for-form-aqueous-ions-for-example-mathrm-ca-left-ma-pa202
(Solved): 1) Soluble compounds dissolve in water for form aqueous ions. For example: \[ \mathrm{Ca}\left(\ma ...
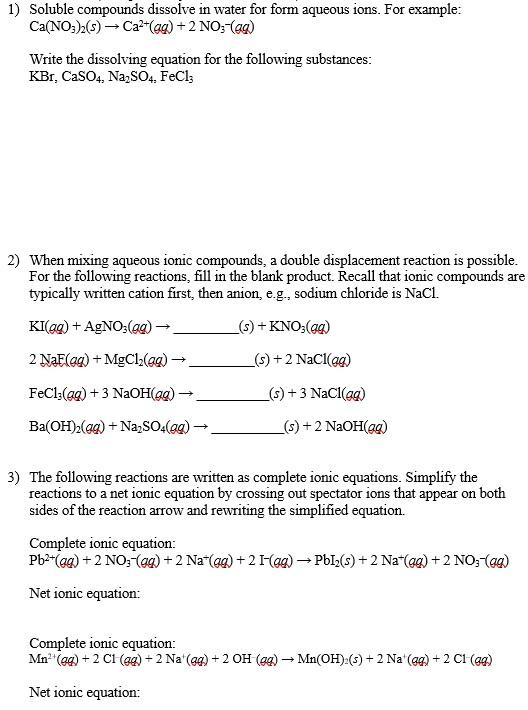
1) Soluble compounds dissolve in water for form aqueous ions. For example: \[ \mathrm{Ca}\left(\mathrm{NO}_{3}\right)_{2}(s) \rightarrow \mathrm{Ca}^{2+}(a q)+2 \mathrm{NO}_{3}^{-}(a q) \] Write the dissolving equation for the following substances: \[ \mathrm{KBr}, \mathrm{CaSO}_{4}, \mathrm{Na}_{2} \mathrm{SO}_{4}, \mathrm{FeCl}_{3} \] 2) When mixing aqueous ionic compounds, a double displacement reaction is possible. For the following reactions, fill in the blank product. Recall that ionic compounds are typically written cation first, then anion, e.g., sodium chloride is \( \mathrm{NaCl} \). \[ \begin{array}{l} \mathrm{KI}(a q)+\mathrm{AgNO}_{3}(a q) \rightarrow \\ 2 \mathrm{NaE}(a q)+\mathrm{MgCl}_{2}(a q) \rightarrow \\ \mathrm{FeCl}_{3}(a q)+3 \mathrm{NaOH}(a q) \rightarrow \\ \mathrm{Ba}(\mathrm{OH})_{2}(a q)+\mathrm{Na}_{2} \mathrm{SO}_{4}(a q) \rightarrow \end{array} \] \[ (s)+\mathrm{KNO}_{3}(q q) \] \[ (s)+2 \mathrm{NaCl}(a q) \] \[ (s)+3 \mathrm{NaCl}(a q) \] \[ (s)+2 \mathrm{NaOH}(q q) \] 3) The following reactions are written as complete ionic equations. Simplify the reactions to a net ionic equation by crossing out spectator ions that appear on both sides of the reaction arrow and rewriting the simplified equation. Complete ionic equation: \[ \mathrm{Pb}^{2+}(q q)+2 \mathrm{NO}_{3}-(q q)+2 \mathrm{Na}^{+}(g q)+2 \mathrm{I}-(g q) \rightarrow \mathrm{PbI}_{2}(s)+2 \mathrm{Na}^{+}(g q)+2 \mathrm{NO}_{3}^{-}(q q) \] Net ionic equation: Complete ionic equation: \[ \mathrm{Mn}^{2+}(g q)+2 \mathrm{Cl}(g q)+2 \mathrm{Na}^{+}(g q)+2 \mathrm{OH}(a q) \rightarrow \mathrm{Mn}(\mathrm{OH})_{2}(s)+2 \mathrm{Na}^{+}(g q)+2 \mathrm{Cl}(g q) \] Net ionic equation: