Home /
Expert Answers /
Chemical Engineering /
1-methane-is-burned-with-atmospheric-air-the-analysis-of-the-products-on-a-dry-basis-is-as-follow-pa608
(Solved): 1- Methane is burned with atmospheric air. The analysis of the products on a dry basis is as follow ...
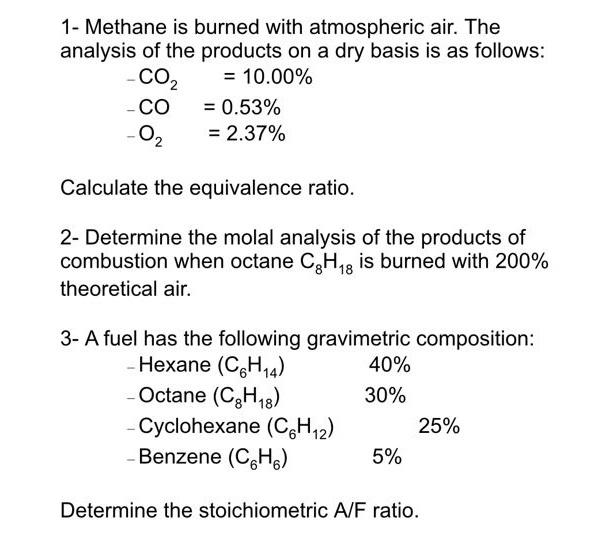
1- Methane is burned with atmospheric air. The analysis of the products on a dry basis is as follows: Calculate the equivalence ratio. 2- Determine the molal analysis of the products of combustion when octane is burned with theoretical air. ?n: Determine the stoichiometric ratio.
Expert Answer
1) To calculate the equivalence ratio, we need to ...