Home /
Expert Answers /
Chemistry /
1-employing-the-huckel-rule-clearly-show-how-both-molecules-a-and-b-are-both-aromatic-a-b-huckel-pa305
(Solved): 1. Employing the Huckel rule, clearly show how both molecules A and B are both aromatic. A B Huckel ...
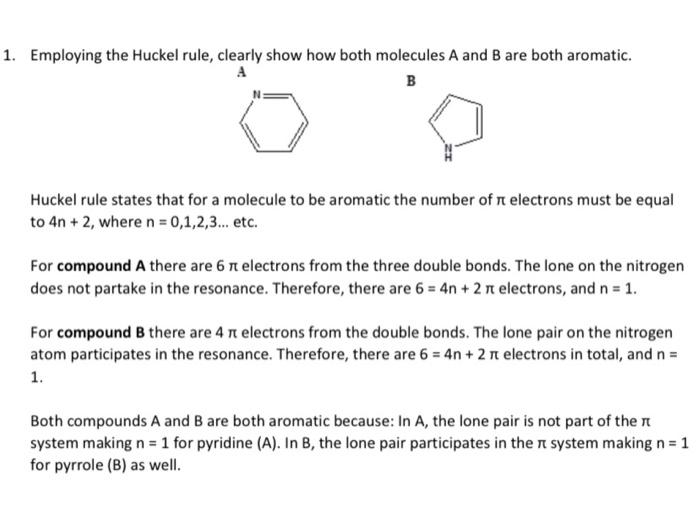
1. Employing the Huckel rule, clearly show how both molecules A and B are both aromatic. A B Huckel rule states that for a molecule to be aromatic the number of electrons must be equal to 4n + 2, where n = 0,1,2,3... etc. For compound A there are 6 ? electrons from the three double bonds. The lone on the nitrogen does not partake in the resonance. Therefore, there are 6 = 4n+ 2 ? electrons, and n = 1. For compound B there are 4 r electrons from the double bonds. The lone pair on the nitrogen atom participates in the resonance. Therefore, there are 6 = 4n+ 2 ? electrons in total, and n = 1. Both compounds A and B are both aromatic because: In A, the lone pair is not part of the system making n = 1 for pyridine (A). In B, the lone pair participates in the system making n = 1 for pyrrole (B) as well.
Employing the Huckel rule, clearly show how both molecules and are both aromatic. B Huckel rule states that for a molecule to be aromatic the number of electrons must be equal to , where etc. For compound there are electrons from the three double bonds. The lone on the nitrogen does not partake in the resonance. Therefore, there are electrons, and . For compound B there are electrons from the double bonds. The lone pair on the nitrogen atom participates in the resonance. Therefore, there are electrons in total, and 1. Both compounds A and B are both aromatic because: In A, the lone pair is not part of the system making for pyridine (A). In , the lone pair participates in the system making for pyrrole (B) as well.