Home /
Expert Answers /
Chemistry /
1-calculate-the-concentration-of-a-solution-prepared-by-adding-15-00ml-of-2-04103mkmno-from-pa537
(Solved): 1. Calculate the concentration of a solution prepared by adding 15.00mL of 2.04103MKMnO from ...
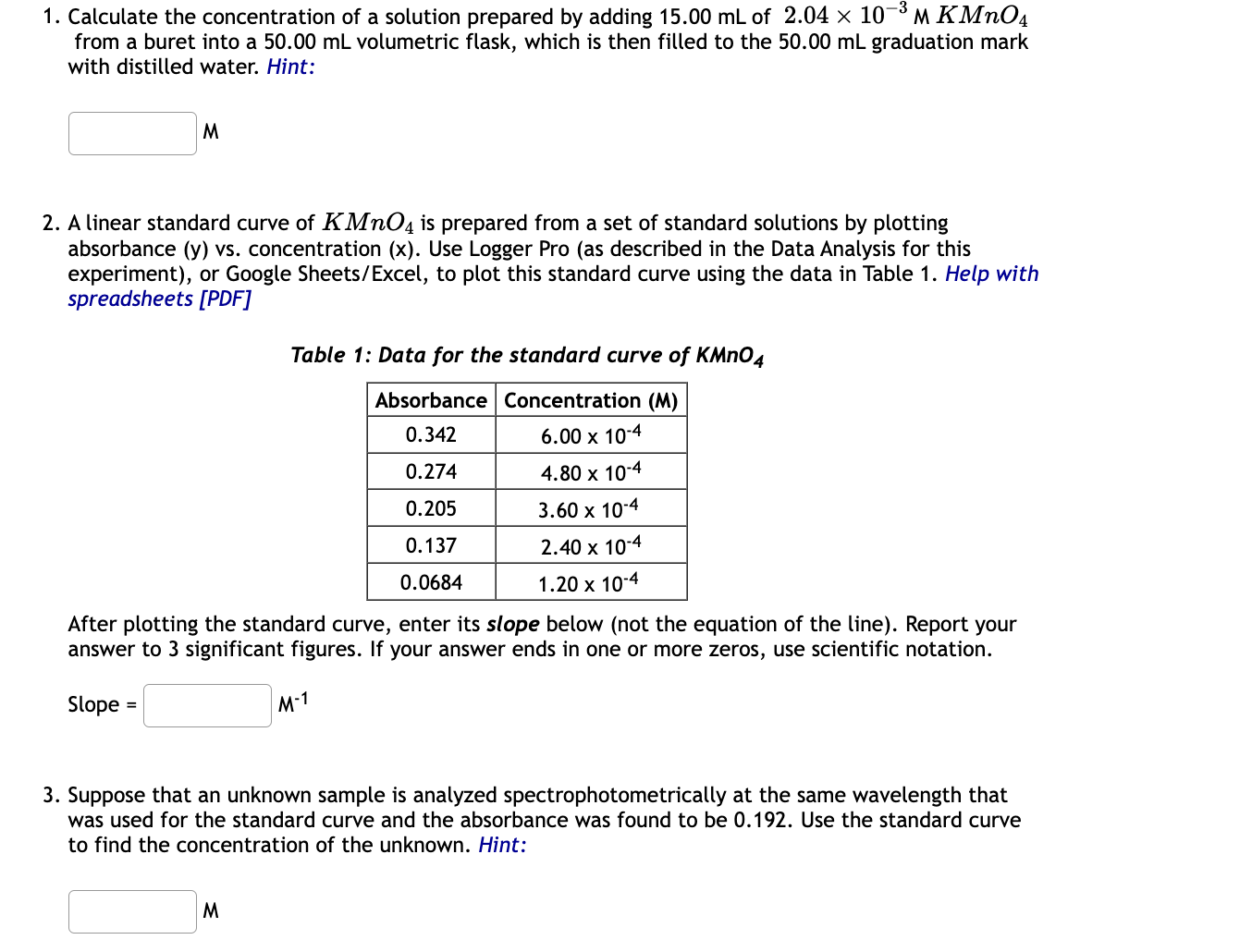
1. Calculate the concentration of a solution prepared by adding of from a buret into a volumetric flask, which is then filled to the graduation mark with distilled water. Hint: M 2. A linear standard curve of is prepared from a set of standard solutions by plotting absorbance (y) vs. concentration (x). Use Logger Pro (as described in the Data Analysis for this experiment), or Google Sheets/Excel, to plot this standard curve using the data in Table 1. Help with spreadsheets [PDF] Table 1: Data for the standard curve of After plotting the standard curve, enter its slope below (not the equation of the line). Report your answer to 3 significant figures. If your answer ends in one or more zeros, use scientific notation. Slope 3. Suppose that an unknown sample is analyzed spectrophotometrically at the same wavelength that was used for the standard curve and the absorbance was found to be 0.192 . Use the standard curve to find the concentration of the unknown. Hint: