Home /
Expert Answers /
Chemistry /
1-calculate-e-and-e0-for-the-following-cell-at-25c-assuming-ideal-solutions-and-no-transfer-of-pa241
(Solved): 1. Calculate E and E0 for the following cell at 25C, assuming ideal solutions and no transfer of ...
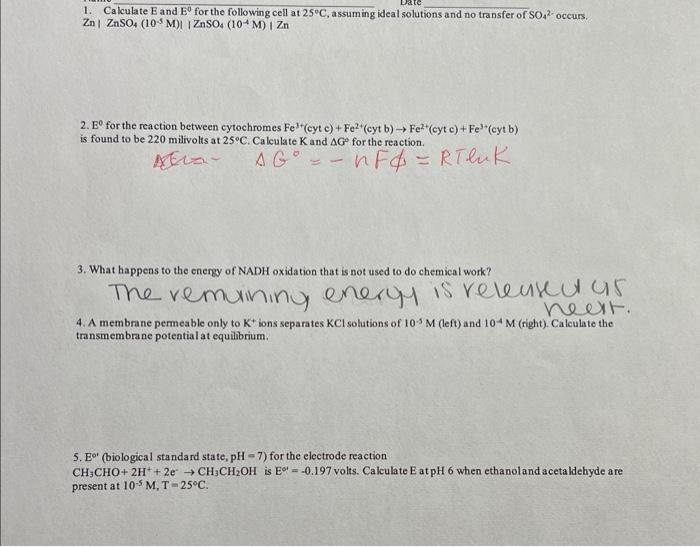
1. Calculate and for the following cell at , assuming ideal solutions and no transfer of occurs. 2. for the reaction between cytochromes cyt c (cyt b) (cyt c) (cyt b) is found to be 220 milivolts at . Calculate and for the reaction. 3. What happens to the energy of NADH oxidation that is not used to do chemieal work? The remuining energy is releukeulas 4. A membrane permeable only to ions separates solutions of (left) and (right). Calculate the transmembrane potential at equilibrium. 5. (biological standard state, ) for the electrode reaction is volts. Calculate at when ethanoland acetaldehyde are present at .