Home /
Expert Answers /
Chemistry /
1-balance-the-following-chemical-reactions-a-na2s-aq-mgcl2-aq-nacl-aq-mgs-s-wo2-pa451
(Solved): 1. Balance the following chemical reactions. a. Na2S(aq)+MgCl2(aq)NaCl(aq)+MgS(s)WO2 ...

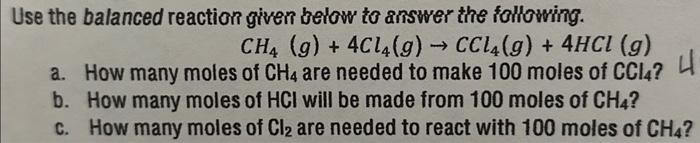
1. Balance the following chemical reactions. a. b. c. d. e. f. g. h. l. j. 2. Use the balanced reaction given below to answer the following. a. How many moles of are needed to make 20 moles of b. How many moles of will be produced when 20 moles of . reaction? 3. Use the balanced reaction given below to answer the following. a. How many moles of are needed to react with 5.25 moles b. How many moles of are produced by the complete com? ? c. How many moles of are produced by the complete com ? 4. Use the balanced reaction given below to answer the following
Use the balanced reaction given below to answer the following. a. How many moles of are needed to make 100 moles of ? b. How many moles of will be made from 100 moles of ? c. How many moles of are needed to react with 100 moles of ?