Home /
Expert Answers /
Chemistry /
1-atomic-mass-amu-2-d-estimate-the-approximate-weight-of-each-and-then-complete-the-calculation-pa729
(Solved): 1. Atomic Mass (amu) 2. D Estimate the approximate weight of each and then complete the calculation ...
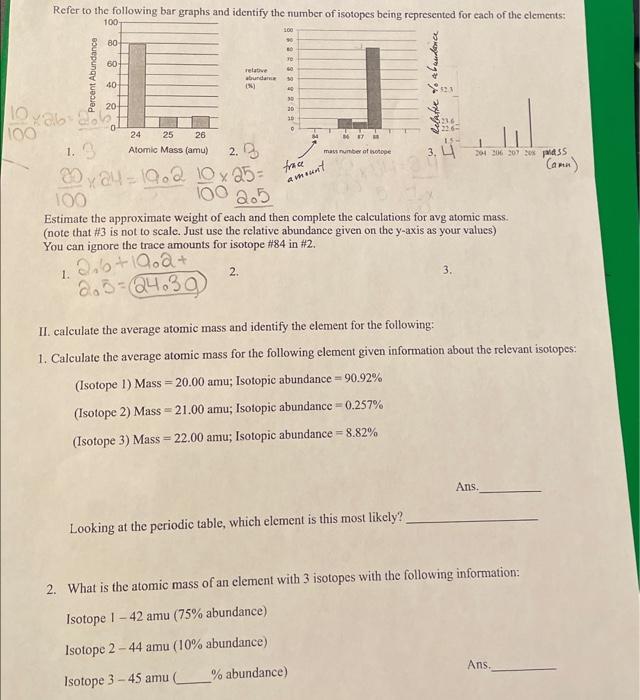
1. Atomic Mass (amu) 2. D Estimate the approximate weight of each and then complete the calculations for avg atomic mass. (note that is not to scale. Just use the relative abundance given on the -axis as your values) You can ignore the trace amounts for isotope in . 1. 2. 3. II. calculate the average atomic mass and identify the element for the following: 1. Calculate the average atomic mass for the following element given information about the relevant isotop (Isotope 1) Mass ; Isotopic abundance (Isotope 2) Mass ; Isotopic abundance (Isotope 3) Mass Isotopic abundance Ans. Looking at the periodic table, which element is this most likely? 2. What is the atomic mass of an element with 3 isotopes with the following information: Isotope I- 42 amu ( abundance) Isotope 2 - 44 amu ( abundance) Isotope amu abundance) Ans.